Targeted protein degradation (TPD) approaches represent a breakthrough in drug development. Unlike traditional occupancy-driven inhibitors, TPD exploits the ubiquitin-proteasome system (UPS) to eliminate disease-causing proteins that were previously considered “undruggable.” Currently, several TPD drugs have shown clinical potential in treating many diseases. However, with extended treatment, patients often develop resistance characterized by downregulation of E3 ligase expression or mutations in UPS components, severely limiting the long-term efficacy of TPD therapies. Therefore, overcoming resistance and enhancing TPD drug efficacy remain urgent challenges.
In January, the research team led by Professor Cang Yong from the School of Life Science and Technology (SLST) at ShanghaiTech University, in collaboration with the team led by Dr. Lu Wei from the Hematology Department of Shanghai Ninth People’s Hospital, published a paper titled “Inhibition of mTOR Enhances the Efficacy of Proteasome-Dependent Targeted Protein Degradation Approaches” in Cancer Research. The study reveals the critical role of the mTOR signaling pathway in regulating the efficacy of proteasome-dependent TPD approaches, and proposes a novel clinical translation strategy to overcome TPD drug resistance through the combination with mTOR inhibitors.
To identify key regulators that can enhance TPD efficacy, the team performed three genome-wide CRISPR screens and identified the mTOR signaling pathway as a critical determinant. mTOR plays a central role in cellular metabolism and is frequently aberrantly activated in various malignancies. The researchers found that activation of mTOR weakens TPD efficiency, while inhibition of mTOR signaling significantly enhances the efficacy of multiple proteasome-dependent degraders tested, including molecular glue degraders (MGDs) and PROTACs.
Mechanistically, mTOR inhibition suppressed de novo protein synthesis, thus creating a synthetic vulnerability by depleting replenishment of proteins targeted by PROTACs or MGDs. (Figure 1).

Figure 1. A schematic of the mechanism. mTOR inhibitors suppress protein synthesis and synergistically enhance the clearance efficiency of TPD drugs on target proteins.
To evaluate the clinical translational potential of this strategy, the team conducted studies in multiple myeloma (MM) models. MM is a hematologic malignancy characterized by the proliferation of abnormal plasma cells.Immunomodulatory drugs IMiDs, a class of MGDs, form the therapeutic backbone of treatment. The study revealed that mTOR inhibitors restored the sensitivity to pomalidomide in pomalidomide-resistant myeloma cell lines. Importantly, in the clinical trial of mTOR inhibitor combined with pomalidomide treatment in patients with relapsed/refractory multiple myeloma, the combination therapy effectively reduced the number of malignant plasma cells in patients and significantly improved multiple clinical parameters. Furthermore, the clinical trial was the first to demonstrate that the combination treatment delayed the emergence of drug resistance and suppressed disease relapse in multiple myeloma (Figure 2).
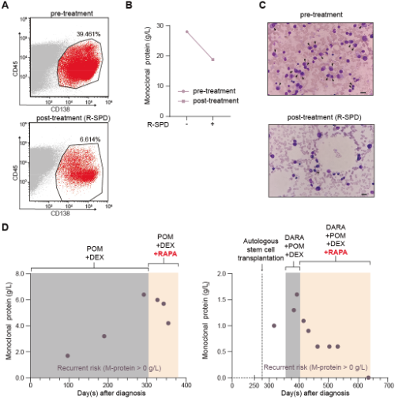
Figure 2. Combination therapy with mTOR inhibitors improves clinical parameters and delays relapse in patients with relapsed/refractory multiple myeloma.
This study highlights that modulating the cellular proteostasis network through the mTOR pathway can substantially expand the therapeutic window of TPD approaches. It not only provides a new perspective on TPD resistance mechanisms but also offers direct theoretical support and therapeutic strategies for clinically enhancing the efficacy of MGDs or PROTACs by combining with already approved mTOR inhibitors, holding significant translational potential.
The co-first authors of the paper are 2020 PhD student Liu Yang and Postdoctoral Fellow Song Tianyu from Cang’s group and Postdoctoral Fellow Sun Yifeng from the Hematology Department of Shanghai Ninth People’s Hospital. The co-corresponding authors are Professor Cang Yong, Dr. Lu Wei, and Song Tianyu. ShanghaiTech University is the first affiliation.
*This article is provided by Prof. Cang Yong

