On December 23, Assistant Professor Xi Ying’s lab at the School of Life Science and Technology (SLST), in collaboration with Prof. Ren Tao from Shanghai Sixth People’s Hospital and Prof. Zhao Jincun from Guangzhou Medical University/Guangzhou Laboratory, published a paper entitled “Dysplastic epithelial repair promotes the tissue-residence of lymphocytes to inhibit alveolar regeneration post viral infection” in the journal Cell Stem Cell. For the first time, this work reveals the pathogenetic role of KRT5+ pods in lung regeneration, serving as a niche for tissue-resident lymphocytes that specifically inhibit airway club cell mediated-alveolar repair.
Severe viral infections, including those caused by pandemic influenza virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can lead to extensive damage to the alveolar epithelium, resulting in diffuse alveolar damage and potential lung failure. In response, regional epithelial stem/progenitor cells are activated to repair and regenerate the alveoli. However, a dysplastic repair represented by KRT5+ basal-like cells can also occur. While KRT5+ basal-like cells rapidly reestablish the epithelial barrier, they lack gas-exchange capacity and consequently impede functional lung recovery. However, the process by which they impair alveolar regeneration remains unclear. Is it due to their mere presence in the alveolar space, or do they actively participate in pathogenic processes?
Respiratory viral infection triggers innate and adaptive antiviral host responses, with timely and robust T cell activation being crucial for virus control. However, prolonged or dysregulated T cell activity after viral clearance may contribute to lung pathology. The authors revealed co-localization of KRT5+ basal-like cells with CD4+ and CD8+ T cells in the lungs of influenza-injured mice, as well as in lung tissues from IPF and COVID-19 patients. Live-cell imaging within the organoid cocultures recapitulated the recruitment of CD4+ effector T cells and CD8+ T cells by KRT5+ cells. Further validation in organoids and in vivo demonstrated that KRT5+ cells promote T cell recruitment and lung tissue retention through pathways including CXCL10/11-CXCR3, Integrins α4β7, and Integrins αL/αM/αX/αDβ2.
This study reveals that tissue-resident T cells inhibit the differentiation of airway club cells into AT2 cells by secreting IFNγ, thereby impeding alveolar repair. Depletion of CD4+ or CD8+ T cells, blockade of IFNγ, CXCR3 or Integrin α4β7, or specific inhibition of IFNγ signaling in Club cells all alleviated this suppression and promoted Club cell-mediated alveolar regeneration.
Collectively, these findings demonstrate that aberrantly repaired KRT5+ basal-like cells actively suppress alveolar repair and regeneration—not merely by occupying alveolar space—but by promoting T cell tissue retention. This research provides a theoretical framework for understanding how maladaptive repair impedes alveolar regeneration, and identifies IFNγ, CXCR3, and Integrin α4β7 as potential therapeutic targets for promoting post-infection alveolar regeneration.
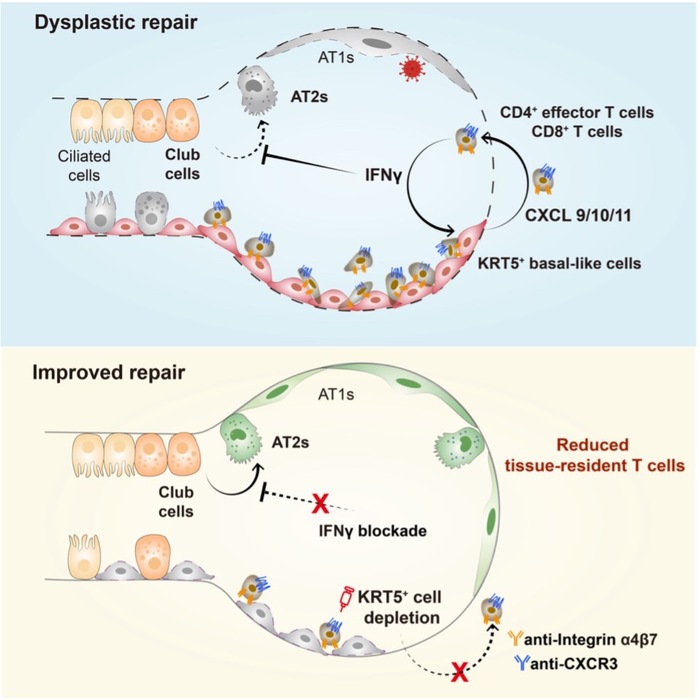
Figure 1. Graphic abstract of the study
Research Assistant Professor Lu Tiantian and 2022 PhD students Liu Li and Wang Ping are the co-first authors. Profs. Xi Ying, Ren Tao, and Zhao Jincun are the co-corresponding authors. ShanghaiTech is the first affiliation.
*This article is provided by Prof. Xi Ying

