Recently, the research team led by Assistant Professor Sun Zhaoru from the School of Physical Science and Technology (SPST) at ShanghaiTech University achieved a major advance in understanding the dissociation and proton transfer mechanisms of carbonic acid in aqueous solutions. The team has long been dedicated to developing novel machine learning force fields that combine first-principles accuracy with the efficiency of classical molecular dynamics to overcome the theoretical simulation bottleneck of dynamic processes in complex solution systems. Using deep potential molecular dynamics (DPMD), they discovered for the first time that the cis-trans (CT) conformer of carbonic acid (H2CO3), which accounts for only 12.4% of the solution, contributes 58.1% of the protons. The study elucidated the reasons behind the CT conformer’s strong proton-donating ability from both hydrogen-bond topological networks and electronic structure perspectives. Furthermore, it revealed the quantum mechanical regulatory mechanism of the solution environment on the dynamic equilibrium of molecular conformations, providing critical theoretical support for applications such as physiological pH regulation and ocean acidification prediction. The findings were published in the prestigious journal Science Advances.
Carbonic acid, a key proton buffer in biological fluids and marine environments, regulates acid-base equilibrium through the dynamic balance of three conformers: cis-cis (CC), CT, and trans-trans (TT). While previous studies have reached a consensus on the stability advantage of the CC conformer in the gas phase, the stability order of conformers in aqueous solutions has long been debated. Additionally, the microscopic mechanisms behind the dissociation of polyprotic weak acids remain poorly understood.
Zhao Yueqi, a master’s student in Prof. Sun’s group, innovatively employed DPMD to simulate carbonic acid dissociation over hundreds of nanoseconds. By comparectronic structures and hydrogen-bond networks in gas and liquid phases, the team made groundbreaking discoveries:
Hydrogen-bond topology governs proton transfer
Although the CC conformer dominates in solution, accounting for 81.3%, the CT conformer participates in more hydrogen-bonded ring structures, enabling more potential proton transfer pathways. As a result, it serves as the primary proton donor, contributing 58.1% of protons.
Electronic asymmetry drives dissociation
The aqueous environment induces significant electronic asymmetry in the CTonformer’s hydroxyl groups. Compared to CC, the lone pair electrons in CT are 0.12 Å farther from the nucleus, while bonding electron pairs are 0.12 Å closer. This enhanced hydrophilicity reduces hydroxyl stability, increasing dissociation probability.
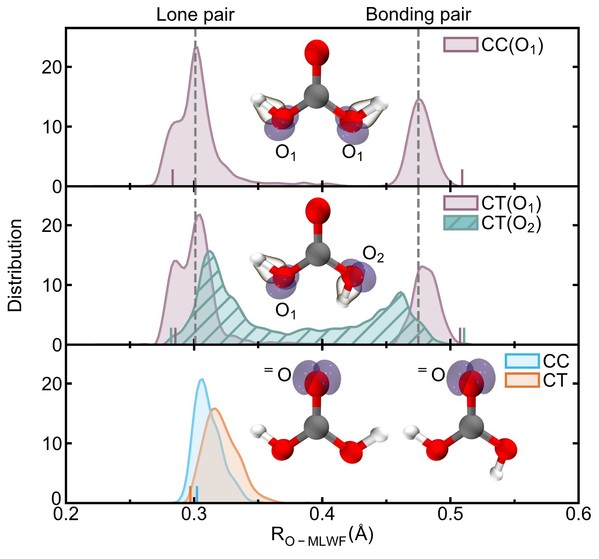
Electronic structures of CC and CT conformers in solution
Proton transfer pathway preference
Protons from CT dissociation exhibit an 82.95% preference for the “returning path,” compared to only 57.76% for CC. This stems from a greater difference in proton competition ability between the deprotonated oxygen and the original carbonyl oxygen in CT-derived HCO3−.
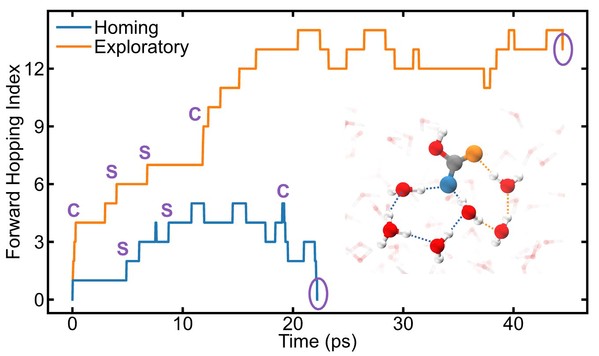
Proton transfer pathways in carbonic acid solution
This study is the first to reveal the microscopic mechanism behind CT’s proton-donating dominance through hydrogen-bond topology and electronic structure analysis, while also resolving the long-standing debate on conformer stability in solution. The findings not only provide an atomic-scale theoretical framework for proton transfer in enzymatic catalysis and dynamic pH regulation in physiological environments but also establish a foundation for modeling ocean acidification kinetics.
Zhao Yueqi, the 2022 master’s student from SPST, is the first author and Tian Feifei, the 2023 master’s graduate, is the second author. Prof. Sun Zhaoru is the sole corresponding author, and ShanghaiTech University is the sole affiliation.
*This article is provided by Prof. Sun Zhaoru

