High cholesterol is a major risk factor for atherosclerosis, heart disease, and stroke. According to the World Health Organization (WHO), over 4.4 million people die annually from cardiovascular diseases linked to high cholesterol, posing a significant public health challenge. Understanding how the body regulates cholesterol levels is crucial for disease prevention and drug development. Sterol Regulatory Element-Binding Protein 2 (SREBP2) is a key regulator of cholesterol synthesis. When cholesterol is scarce, SREBP2 is released from its membrane-bound precursor, enters the nucleus, and activates cholesterol synthesis genes. Discovered 30 years ago by Nobel laureates Michael Brown and Joseph Goldstein, the precise mechanism by which nuclear SREBP2 (nSREBP2) activates these genes has remained elusive.
On May 20, Associate Professor Qi Wei’s team from the School of Life Science and Technology (SLST) at ShanghaiTech Univeristy published a study in Nature Metabolism, titled “Nuclear SREBP2 condensates regulate the transcriptional activation of lipogenic genes and cholesterol homeostasis.” The research reveals how nSREBP2 forms nuclear condensates through phase separation—a process akin to oil droplets clustering—acting like a “molecular factory” to drive cholesterol synthesis.
When cells lack cholesterol, nSREBP2 undergoes phase separation to form nuclear condensates, resembling a “molecular factory” that activates cholesterol synthesis genes. A critical component, the N-terminal intrinsically disordered region (PrLD2 domain) containing phenylalanine 178 (F178), acts as “magic glue.” Mutating F178 (F178A) disrupts this “glue,” dismantling the condensates, impairing gene activation, and reducing cholesterol synthesis. Remarkably, adding a “backup glue” from the FUS protein restores condensate formation, confirming its essential role in cholesterol synthesis.
The team engineered mice with a mutated SREBP2 “glue” site (Phe167, equivalent to human F178). In fasting-refeeding experiments, these mice showed significantly lower liver and blood cholesterol levels compared to normal mice, underscoring the “glue’s” role in cholesterol synthesis.
This study unveils how nSREBP2’s nuclear condensates regulate cholesterol homeostasis, offering insights into metabolic balance. Targeting these condensates could lead to novel therapies for lowering cholesterol and preventing heart disease and other diseases driven by high cholesterol. Additionally, a human SREBP2 variant (P183S) may alter condensate formation, suggesting avenues for personalized medicine.
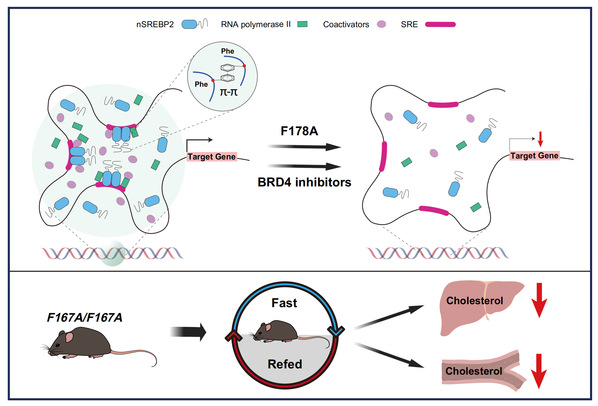
Graphic summary of transcription activation by nSREBP2 condensates.
Doctoral student Xu Mengqiang from Prof. Qi’s group and Jiang Shiyou (formerly a ShanghaiTech visiting scholar) from Kunming Institute of Botany, CAS are the co-first authors, with Prof. Qi Wei as corresponding author. Collaborators include Professor Song Baoliang from Wuhan University and Assistant Professor Ma Hanhui from SLST.

