Led by tenure-track Assistant Professor Hua Tian and Executive Director Liu Zhi-Jie from iHuman Institute at ShanghaiTech University, a joint research team has published a study in Cell Research, revealing how human G protein-coupled receptors (GPCRs) detect changes in acidity (pH). The research focuses on proton-sensing GPCRs, specifically GPR4 and GPR65, uncovering their roles in monitoring and potentially regulating the micro-environments of immunity, inflammation, and cancer. This discovery offers new hope for developing drugs to treat related diseases.
GPCRs are the body’s “signal sensors,” detecting extracellular changes and relaying messages into the cells. About 40% of drugs target these sensors to treat diseases conditions by modulating their activity. GPR4 and GPR65 are unique GPCRs that act as “acid sensors,” detecting pH changes to maintain acid-base balance, regulate immune responses, and influence tumor growth. Until now, how these sensors “switch on” in acidic environments remained a mystery, hindering drug development.
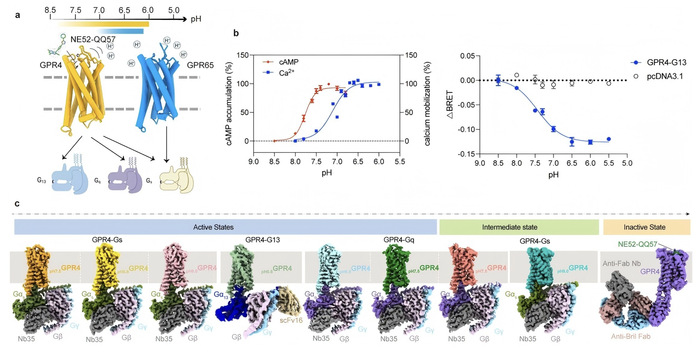
The team discovered that GPR4 and GPR65 use histidine residues (amino acid “probes”) and carboxylic acid residues (acidic chemical groups) to sense acidity. These probes act like “smart switches,” activating in acidic conditions to trigger cellular signals. The study captured a “stepwise activation” process: in mildly acidic conditions, some probes turn on, partly opening the signal pathway; in stronger acidity, the extracellular loop 2 (ECL2), an “antenna-like” structure, shifts from a “flat” to an “upright” β-sheet conformation, fully opening the signal pathway. Excitingly, a molecule called NE52-QQ57 can “lock” GPR4, preventing it from working, offering a precise target for designing selective drugs.
Molecular mechanism for the activation of GPR4 and GPR65
Using single-particle cryo-electron microscopy (cryo-EM) method, the team captured high-resolution “3D snapshots” of GPR4 and GPR65 at pH levels from 6.0 to 8.0, showing how they interact with G proteins (Gs, Gq, G13). Cell-based experiments confirmed their function, while molecular dynamic simulations analyzed how molecules interact, like watching them “shake hands” to pass signals.
This study maps how “acid sensors” work, shedding light on immunity and cancer mechanisms. By revealing the stepwise activation of GPR4 and GPR65 and identifying NE52-QQ57’s unique binding site, it lays the foundation for designing precise drugs that can “switch” these sensors on or off. Such drugs show potentials for treatments of inflammation, cancer, and other pH-related diseases.
Yue Xiaolei, a 2023 PhD student from iHuman Institute and the School of Life Science and Technology, and 2022 PhD students Peng Li and Liu Shenhui are the co-first authors. Prof. Liu Zhi-Jie, Prof. Hua Tian, Research Associate Professor Wu Lijie at iHuman Institute, and Professor Xu Huji from Shanghai Changzheng Hospital are the co-corresponding authors. ShanghaiTech is the first affiliation.

