Led by Professor Liu Ji-Long in the School of Life Science and Technology, a research team has recently made strides in comprehending the structure and inhibition mechanisms of prokaryotic cytidine triphosphate (CTP) synthase, as detailed in the academic journal mLife in a paper entitled “Filamentation and inhibition of prokaryotic CTP synthase with ligands” on May 2. This study provides novel insights into CTP synthase regulation within biological systems and lays groundwork for innovative therapeutic approaches against bacterial pathogens.

CTP synthase (CTPS) is critical in CTP biosynthesis, and is a fundamental precursor for RNA and DNA synthesis. The enzyme interacts directly with all four ribonucleoside triphosphates (ATP, UTP, CTP, and GTP) and participates in the formation of cytoophidia and metabolic fibers which are regulated at various levels within the cell. Utilizing cryo-electron microscopy, the research team resolved the structure of Escherichia coli CTP synthase (ecCTPS) in complex with CTP, reduced nicotinamide adenine dinucleotide (NADH), and a covalent inhibitor, 6-Diazo-5-oxo-L-norleucine (DON), achieving a resolution of 2.9 Å. By constructing a phylogenetic tree based on the differences in metabolic fiber formation, the team discovered the significance of helix 12 in CTPS metabolic fiber formation and created a variant to validate their hypothesis. This underscored the critical role of helix 12 in CTPS metabolic filamentation, offering an evolutionary perspective on the phenomenon. Binding with DON created an accessible ammonia tunnel within ecCTPS and highlighted notable differences in CTP binding modes between prokaryotic and eukaryotic CTPS. Through biochemical and structural analyses, researchers identified a synergistic inhibitory effect of CTP and adenine on ecCTPS with NADH.
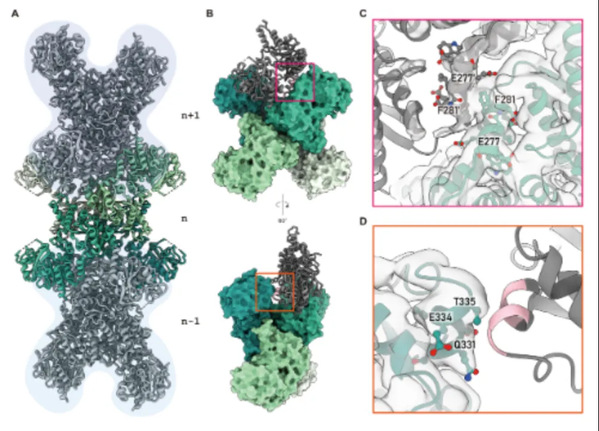
This study enhanced comprehension of diverse CTPS regulatory mechanisms and laid a scientific foundation for designing specific inhibitors targeting bacterial CTPS. The findings hold potential clinical applications and may aid in developing novel therapeutics for bacterial and viral infections.
Second-year PhD student Guo Chenjun, and Wang Zixuan ’24 are the co-first authors of the paper. Professor Liu Ji-Long is the corresponding author.
*This article is provided by Prof. Liu Ji-Long

