Hepatocellular carcinoma, a highly prevalent malignant tumor in China, accounts for nearly half of the newly diagnosed cases and deaths worldwide annually. Among liver cancer patients, 70-80% are diagnosed with tumor spread and metastasis, rendering them ineligible for surgical intervention. However, a comprehensive understanding of the molecular characteristics specific to metastatic liver cancer is still lacking.
Recently, a new ShanghaiTech study has tried to fill the gap. The study is led by the research team of Assistant Professor Zhang Liye in SLST at ShanghaiTech, in partnership with Dr. Fan Jia, MD, at Zhongshan Hospital, Fudan University, and Shanghai Dunwill Medical Technology Development. In this study, the researchers integrated spatiotemporal multi-omics data from 182 hepatocellular carcinoma (hereafter referred to as HCC) patients, selected from over 20,000 surgical cases at Zhongshan Hospital and Tianjin Medical University Cancer Institute and Hospital. These 182 patients’ cases included paired primary and metastatic samples. Through multi-region sampling and combined multi-omics data analysis, this study depicted the complex tumor evolution of HCC metastasis, and uncovered the initiation timelines, clone patterns in metastatic dissemination, and intra-tumoral heterogeneity, thereby providing an invaluable resource. It also identified associations between the tumor microenvironment that drives metastasis and the mechanisms governing clone selection during the metastatic process. These findings aim to provide a more substantial theoretical basis for precision diagnosis and treatment of HCC metastasis.
A tumor evolution tree with multiple spatial and temporal dimensions was constructed to track the clonal origin of metastatic tumors. The results indicate that: 1) in patients with multiple genetically distinct primary lesions, only the primary tumor with the most metastatic potential will cause cancer cell dissemination; 2) compared to multicentric intrahepatic recurrence (MO-RT) that is independently developed and genetically distinct from the primary lesion, intrahepatic recurrence (IM-RT) with genetic similarity to the primary lesion has a stronger metastatic potential; 3) metastatic foci within the same organ or between different organs can spread to each other. The research team further conducted a study on the cloning patterns of metastatic foci with multi-regional sampling, finding that about 1/4 of metastatic tumors were seeded by multiple distinct subclones. Patients with polyclonal seeding progressed rapidly and were associated with poor clinical outcomes, and activation of the hypoxia signaling pathway may be a key driving factor for polyclonal metastasis.
Compared to prior oncological knowledge, this study signified a substantial breakthrough. Traditionally, metastasis has been considered a terminal event in tumor progression, but this study revealed that 80% of HCC metastases begin at early primary tumor stages based on the genetic divergency between paired primary and metastasis, suggesting that neoadjuvant therapy before and after surgery could help eliminate early-disseminated micro-metastatic tumor cells, potentially reducing the risk of postoperative recurrence. Additionally, while classical theories suggest lower intratumoral heterogeneity in metastatic lesions than in primary lesions due to the existence of evolutionary bottlenecks, comparable levels of heterogeneity were observed in both HCC primary and metastases.
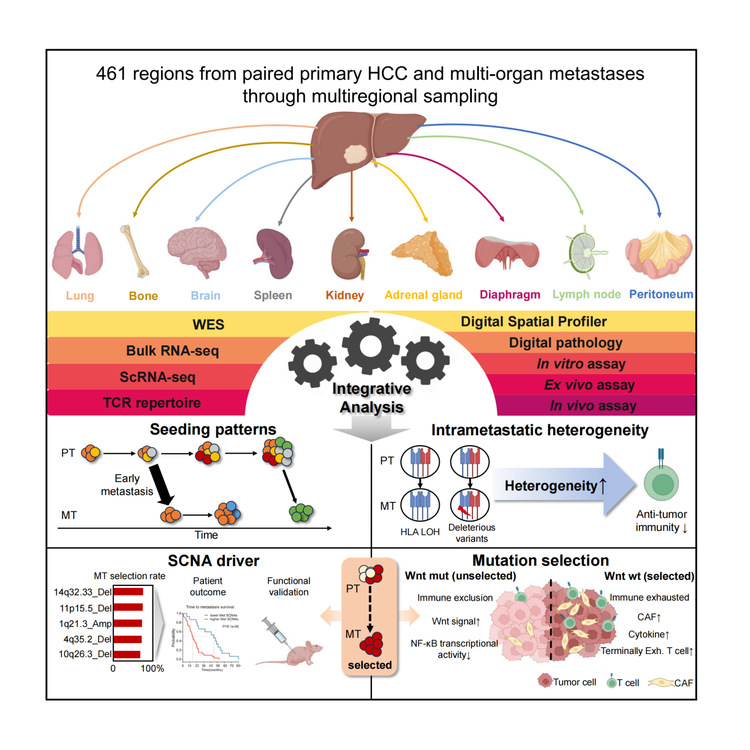
Furthermore, this study elucidated the patterns of tumor subclone dissemination during metastasis. It systematically characterized the evolutionary selection of subclonal driver events and identified seven copy number alterations significantly enriched in metastatic lesions. This also showed the best performance for metastasis prediction. The finding of the study suggests that the competitive advantage of tumor subclones in growth and dissemination during metastasis is not solely based on their migration capacities but also on their ability to shape and utilize the immune microenvironment.
On December 14, this study was published in the prestigious international oncology journal, Cancer Cell, in an article titled “Integrated multi-omics profiling to dissect the spatiotemporal evolution of metastatic hepatocellular carcinoma”. Prof. Zhang Liye, together with Dr. Fan Jia and another two researchers are the corresponding authors of this paper. Dr. Wu Pin and Dr. Wang Zejian at ShanghaiTech are among the co-first authors.
*This article is provided by Prof. Zhang Liye

