In multicellular organisms, the centrosome plays a critical role in development and tissue homeostasis. In various early embryos and stem cells, the centrosome regulates their polarized or asymmetric cell division. Centrosome dysfunction is linked to various diseases including cancer, microcephaly and ciliopathy. APC/C (Anaphase-Promotion Complex/cyclosome) is an evolutionarily conserved ubiquitin ligase complex that regulates cell cycle and coordinates cell differentiation.
A research team led by Assistant Professor Yuu Kimata from the School of Life Science and Technology (SLST) at ShanghaiTech University, in collaboration with Dr. Jens Januscheke at University of Dundee, found that the degradation of Spd2 by cell cycle key regulator APC/C regulates the function of asymmetric centrosome division during mitosis of Drosophila neural stem cells. On February 28, the study was published in a research article in EMBO Reports entitled “APC/C-dependent degradation of Spd2 regulates centrosome asymmetry in Drosophila neural stem cells”.
Inside the Drosophila neuroblasts (NB), two centrosomes show differential microtubule nucleation activity during interphase and this asymmetric activity of centrosomes is important for the division axis maintenance of the NB. Kimata’s research group identifies an evolutionally conserved centrosome component Spd2 (CEP192 in mammals) as a substrate of APC/C ubiquitin ligase—the cell cycle regulating enzyme. Through the live imaging of whole-mount Drosophila larval brains, the results showed that upon Spd2 stabilization or accumulation, both centrosomes lost microtubule nucleation capacity during interphase, and the apical centrosome became detached from the cortex to start wandering around the cytoplasm. In some of the larval brains with higher Spd2 levels, NBs showed significantly larger axis deviations. The daughter centrosomes, which were supposed to be inherited from neural stem cells, were mistakenly divided into a differentiating daughter cell.
Furthermore, the results showed Spd2 accumulation compromises asymmetric PCM loading and increases the mobility of Spd2 that may likely prevent Polo-dependent phosphorylation of Spd2, which is required for the microtubule nucleation activity at one of the daughter centrosomes during interphase.
This study, for the first time, demonstrated the role of ubiquitin-mediated degradation of a conserved PCM protein for stem cell-specific functions of the centrosome. Dysfunction of this mechanism may be implicated in complex human diseases associated with centrosome dysfunctions.
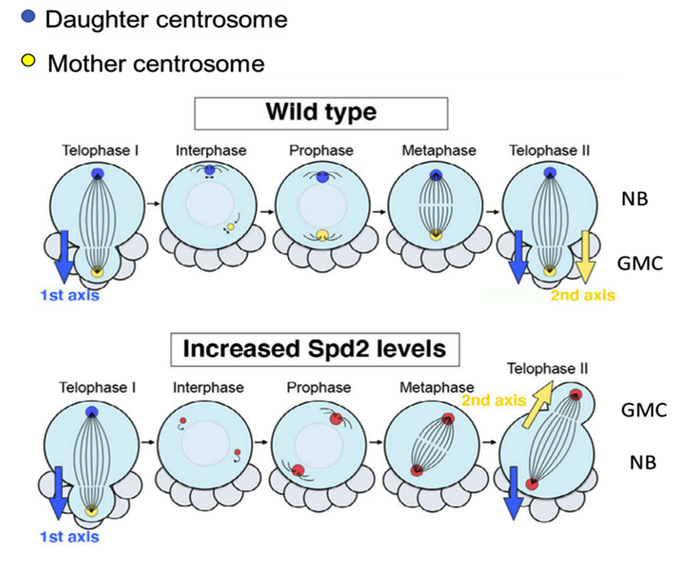
A schematic diagram representing the centrosome behavior during interphase in wild-type larval NBs (neuroblasts) and NBs with increased levels of Spd2.
Ph.D. student Zhang Qian of Kimata's group, and Francesco Meghini and Torcato Martins from University of Cambridge are the co-first authors of this paper. The corresponding author of this paper is Prof. Yuu Kimata of ShanghaiTech University. Research Assistant Yusanjiang Abula of Kimata’s group also participated in the research.

