Organic π-conjugated molecules (OCMs) have become promising candidates in the fields of organic catalysts, bio-imaging/sensing, and organic optoelectronics. Recently, introducing main-group elements (such as B, N, S, Si, P, etc.) into molecular backbones has become a powerful strategy for fine-tuning photo-physics, redox characteristics, molecular organizations, and optoelectronic functionalities of the OCMs. As a special member of the main-group elements, phosphorus (P) element is very effective to tailor the OCMs for specific functionalities due to its rich chemistry.
Azaphosphorus-heterocycles are the P-heterocycles where carbon (C) atom is replaced by nitrogen (N) atom. Due to the different electronic configurations between N atom and C atom, azaphosphorus-heterocycles exhibit distinct chemical structures and reactivities compared with their all-carbon-based counterparts. For example, diazaphosphinine (DP) (in the following figure) exhibits weaker aromatic character, larger dipole moment, and higher reactivity compared with phosphinine. Because of the limited synthetic pathway and/or high reactivity, there are few reports on synthesis, reactivity and theoretical studies of DP derivatives.
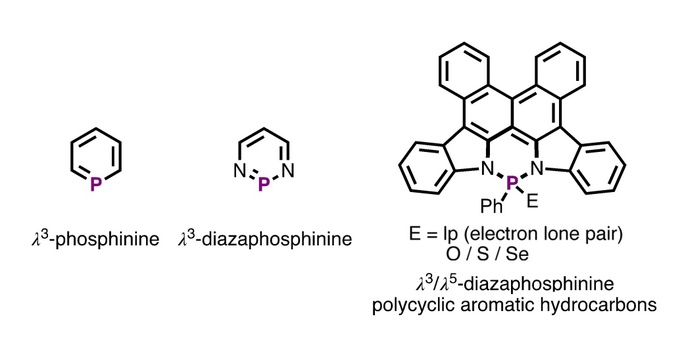
Phosphinine and diazaphosphinine in the literature and diazaphosphinine-based polycyclic aromatic hydrocarbons in this work
In the article entitled “Facile Route to Novel Diazaphosphinine-Based Polycyclic Aromatic Hydrocarbons” published in Angewandte Chemie International Edition, Assistant Professor Ren Yi’s group from SPST reported the first example of diazaphosphinine-based polycyclic aromatic hydrocarbons (DP-PAHs) via facile synthetic protocols that harness efficient phosphorus-nitrogen (PN) chemistry. Furthermore, the heterocycles readily undergo efficient photocyclizations by using only oxidants, which is applicable to the heterocycles with various P-centers. The researchers successfully extended the protocols in extended DP-PAHs with 13-fused aromatic rings. The photophysical and theoretical studies further showed that adjusting P-chemistry allows to efficiently fine-tune the singlet and triplet emission characteristics. More importantly, single-molecule white-light-emitting character was achieved in the crystalline state at room temperature via the balanced singlet and triplet emission of DP-PAH. Overall, this work described a facile strategy to construct new DP-based system, and uncovered the tunable photophysical properties. Therefore, this work opens up new horizons in the area of organic π-conjugated materials.
Graduated master student Yang Zi and third-year master student Li Can are the co-first authors of the paper. Prof. Ren is the corresponding author. ShanghaiTech is the primary affiliation.

