A new study has been reported by the laboratory of Associate Professor Li Xiajun at SLST, ShanghaiTech, to elucidate maintenance of DNA methylation in embryonic stem cells. This paper entitled “DNA methyltransferases are complementary in maintaining DNA methylation in embryonic stem cells” is published in iScience, a Cell press journal, on September 16, 2022.
DNA methylation is the major epigenetic modification on the genetic material DNA that is universally present in living organisms. It has regulatory roles in gene transcription, retroviral silencing, mono-allelic expression of the imprinted genes, chromatin configuration and various human diseases such as cancer. DNA methylation covalent modification is catalyzed by DNA methyltransferase (DNMT). There are three major DNA methyltransferases in mammals (DNMT1, DNMT3A and DNMT3B). Generally speaking, DNMT1 is the major maintenance DNMT to prevent demethylation caused by DNA replication. DNMT3A and DNMT3B are involved in de novo DNA methylation. There are a few reported studies indicating that DNMT1 acts in de novo DNA methylation while DNMT3A and DNMT3B may play some roles in the maintenance DNA methylation. It is still unclear how they may maintain DNA methylation in the genome, particularly at the Imprinting Control Regions (ICRs).
Li’s group had developed an approach to efficiently isolate mouse deletion mutant embryonic stem (ES) cells by CRISPR. A patent application has been filed for this approach. Li’s group used this approach to isolate mouse mutant ES cell clones that lack DNMT1, DNMT3A or DNMT3B. Then they applied combined bisulfite restriction analysis (COBRA) and whole-genome bisulfite sequencing (WGBS) analysis to determine DNA methylation in the repeats, genic regions and other genomic sequences in ES cells.
Three DNA methyltransferases were all required for maintaining DNA methylation in the repeats and genic regions, etc. A number of imprinted genes in mammals and flowering plants exhibit parental origin-dependent mono-allelic expression, which is regulated by the germline-derived differential DNA methylation at the imprinting control region (ICR). Interestingly, DNA methylation at the ICRs that is stably maintained in somatic cells after fertilization was also jointly maintained by three DNMTs in ES cells. Although DNMT1 was the major DNMT for maintaining DNA methylation at most ICRs, DNMT3A and DNMT3B were equally or even more important in maintaining DNA methylation at a subset of ICRs in ES cells than DNMT1. ZFP57, a master regulator in genomic imprinting discovered in Li’s group, recruits DNMT proteins to maintain differential DNA methylation at the ICRs. There are three TET proteins in mammals that are involved in oxidative reaction to remove DNA methylation. Loss of ZFP57 caused loss of DNA methylation at most ICRs in ES cells, which could not be prevented by loss of three TET proteins. These results suggest that active demethylation mediated by TET proteins may not be important for the stable maintenance of DNA methylation at the ICRs in ES cells. These are the new findings of this study that had not been reported previously.
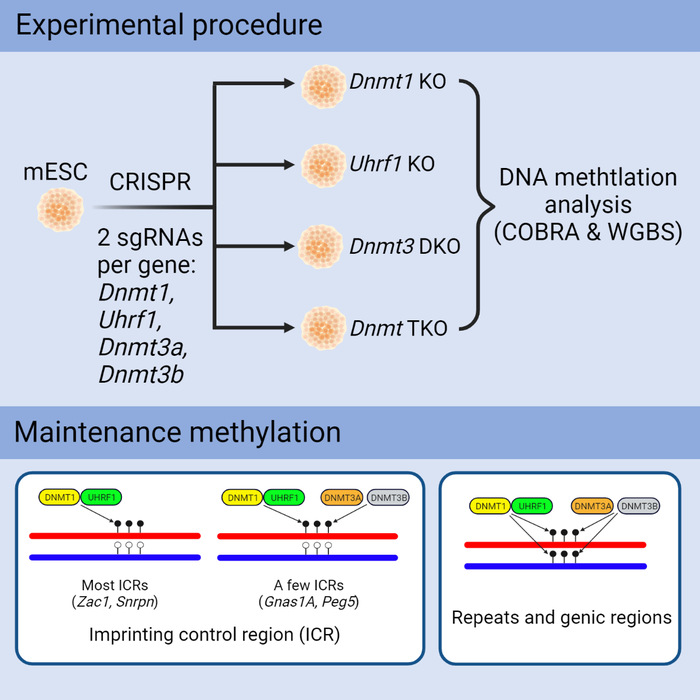
DNA methyltransferases play complementary roles in maintaining DNA methylation in embryonic stem (ES) cells
ES cells and induced pluripotent stem (iPS) cells have multi-potential with almost unlimited proliferation capacities. They can give rise to different cell types that are good candidates for future cell-based therapies for degenerative diseases such as Alzheimer’s disease. Imprinted genes are essential for mammalian development. Improper expression of the imprinted genes causes some major human diseases. The results of this study may help to derive ES cells and iPS cells with stable DNA methylation and normal expression of the imprinted genes for future therapeutic application.
Fifth-year Ph.D. candidate Liu Yuhan, first-year Ph.D. candidate Xu Zhen, third-year Ph.D. candidate Shi Jiajia, and second-year master student Yang Shuting of Li’s group, and Dr. Zhang Yu of Fudan University are the co-first authors. Prof. Li Xiajun is the corresponding author.
*The news article was provided by Prof. Li Xiajun

