In natural silk spinning process, the molecular assembly synergistically realizes the complex and ordered mesoscopic hierarchies. Inspired by this, SPST Ling Shengjie's group cooperated with Yao Yuan's group from ZJU-Hangzhou Global Scientific and Technological Innovation Center, to combine the “molecular-supramolecular assembly” and “phase transition-induced mechanical training” methods to develop a biomimetic meso-assembly-processing engineering (MAPE) strategy which can be applied to construct biomaterials that mimic soft tissue structure and its mechanical properties. The highly hydrated silk protein composites prepared by this strategy have good mechanical properties and can be controlled. Such materials can have high stretchability (failure strain greater than 1200%), and high strength and toughness (strength 5±1 MPa, stiffness 18±2 MPa, toughness 6 MJ m-3). This kind of silk protein material can be mechanically matched with different biological tissues, and in application, it can realize the regulation of cell reconstruction shape and promote the recovery of cell physiological function. This research result has been recently published in the journal Advanced Functional Materials in the article entitled “Natural silk spinning-inspired meso-assembly-processing engineering strategy for fabricating soft tissue-mimicking biomaterials”.
During the natural spinning process, silk proteins are assembled into silk protein globules in silk glands, and nanofibers are formed under the action of stretching and shearing forces, and finally silk fibers with a multi-level mesoscopic structure are formed. In this research, reconstituted silkworm silk fibroin (RSSF)/polyethylene oxide (PEO) was used to simulate the phase separation system in natural spinning dope. During the drying process of the solution, as the concentration increased, the RSSF micelles assembled into spherical structures similar to those in natural silk glands. By adjusting the solution composition ratio or solvent evaporation rate, the diameter of the RSSF globules can be regulated in the range of 0.5-200 μm, and finally biomimetic mesoscopically assembled films (BMAFs) are fabricated. These films are macroscopically uniform and defect-free; while at the mesoscopic scale, they exhibit a clear phase-separated structure.
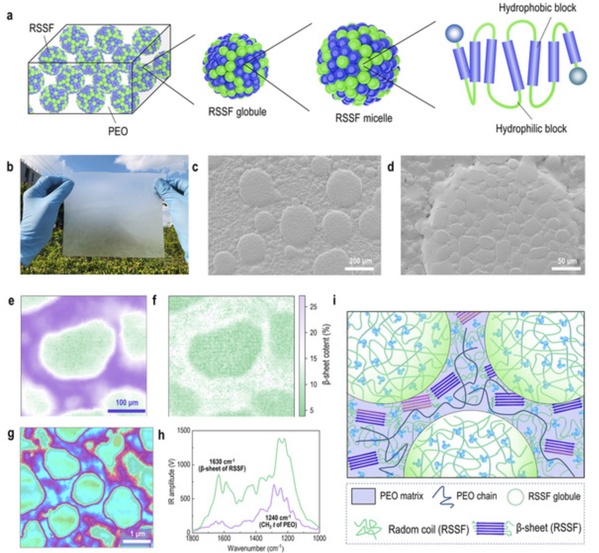
RSSF/PEO-based biomimetic mesoscopic assemblies
Mechanical training (repeated stretching) of BMAFs enables the RSSF structural reconstitution at the mesoscopic scale. Finally, the RSSF globules evolved into nanofibrous structures with diameters of about 300-500 nm, and the morphology was similar to that of nanofibers in natural silk. The results of the microstructural evolution of BMAF during mechanical training showed that after mechanical training, β-strands (β-strands) were parallel to the film plane, that is, oriented along the fibril axis.
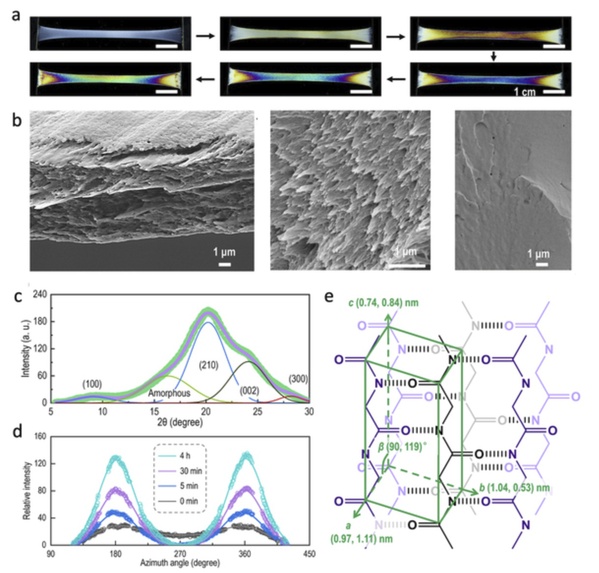
RSSF structural reconstitution after mechanical training to BMAF. (a) BMAF during the mechanical training. (b) Structure of BMAF after training (left) compared with native silk (middle) and pure RSSF film after training (right). (c)-(d) BMAF microstructural evolution after the characterization by wide-angle X-ray scattering and small-angle X-ray scattering
The BMAFs prepared by this method have high water content (59±3 wt%). They exhibit typical mechanical behavior of tough materials, and have a wide range of mechanical characteristics such as flexibility, stretchability, and toughness. It can be matched with different tissues in biological tissue engineering applications, and its comprehensive mechanical properties are better than most hydrogel materials with different matrices and high-water silk protein-based materials.
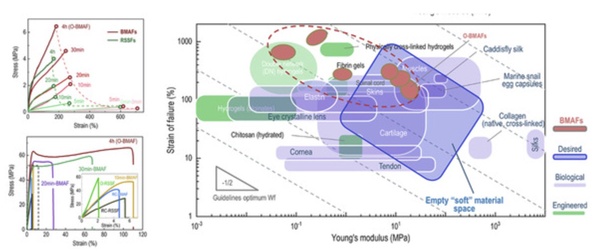
Changes in mechanical properties of BMAFs during training and the comparison with mechanical properties of various biological soft tissues
In biological tissue engineering, the matrix can affect the physiological function of cells by affecting their morphology, so the microstructure and mechanical properties of matrix materials are very important. This study showed that BMAFs had excellent adhesion to 293T cells and HeLa cells, and the anisotropy of cells increased with the degree of orientation of BMAFs.
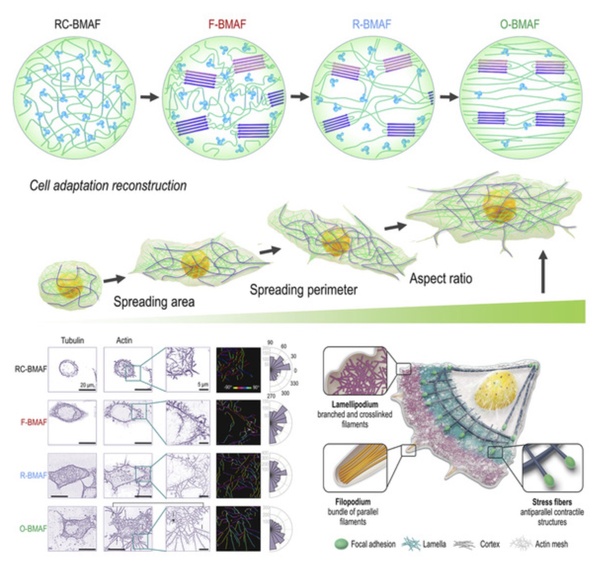
The mesostructure of BMAFs triggers the morphological transition of HeLa cells. The manner in which HeLa cells adjust their structure on BMAFs involves spatial reconstitution of filaments and modulation of aggregation
This study developed a biomimetic MAPE strategy, constructed biomaterials with hierarchical structure and tunable mechanical properties, and explored the role of such biomimetic mesoscopic assembly materials in regulating cell morphology, which can be used for silk protein-based materials. The research results can provide useful reference for the expansion of the application of silk protein based biomaterials.

