On October 20, Assistant Professor Liu Ru-Juan and her colleagues at SLST, together with her collaborators, published a research article entitled “THUMPD3-TRMT112 is a m2G methyltransferase working on a broad range of tRNA substrates” in Nucleic Acids Research. This study reveals that THUMP domain-containing protein 3 (THUMPD3) interacts with an auxiliary protein (methyltransferase activator TRMT112) to broadly modify m2G modification at 6th and 7th nucleotides of tRNAs in eukaryotes. This study not only elucidates the modification mechanism of THUMPD3 and its complexes, but also paves a way for investigating the biological functions of tRNA:m2G.

During gene expression, tRNA recognizes codons on mRNA through its anticodons, and transfers corresponding amino acids to the polypeptide chain synthesized by ribosome. At present, tRNA is the RNA molecule with the most known modification types and the highest modification intensity in cells, and one tRNA molecule carries 7-15 modifications. Although many tRNA modifications were identified decades ago, the study of genes encoding tRNA modifying enzymes lags far behind, and about 25% of human tRNA modifying enzymes are yet to be revealed. In recent years, an increasing number of studies have shown that methylation modification widely existing in tRNAs plays a series of non-classical functions, such as regulating metabolism and immunity in a variety of life activities.
Interestingly, some tRNAs have conserved m2G (N2-methylguanosine) modifications at nucleotide 6 in amino acid acceptors where modification rarely occurs. Recent studies have shown that m2G and m5C modifications of tRNA-derived small RNA fragments (tRFs) are significantly enriched in the sperm of mice with abnormal dietary metabolism, suggesting that these two modifications play an important role in tRFs-mediated paternal intergenerational inheritance. Although the role of tRNA:m5C in mammals has been described in the literature, there are few studies on the mechanism of m2G on tRNAs, especially the identification of m2G modifying enzymes.
In this study, THUMPD3-TRMT112, a methyltransferase complex responsible for the catalysis of tRNA:M2G6/7 in eukaryotes, is reported for the first time, providing new insights into the recognition mechanism of proteins containing the THUMP domain and binding substrates of a broad range of methyltransferase activation helper proteins TRMT112. It is worth mentioning that Liu Ru-Juan and He Chuan (University of Chicago) jointly published a paper in Nature Cell Biology in July this year, revealing that the demodification enzyme Alkbh7 can remove m22G, the methylation product of m2G. Whether or not Alkbh7 and its family members can dynamically regulate tRNA:m2G6/7 remains a question that deserves further attention.
The first author of this paper is Yang Wenqing, a graduate student in Liu’s Lab. Assistant Professor Liu Ru-Juan is the corresponding author. Collaborators from the CAS Center for Excellence in Molecular Cell Science, SIAIS in ShanghaiTech University and Hainan University also contributed to this work. Assistant Professor Yang Bei from SLST/SIAIS and Assistant Professor Liao Jun from SLST provided technical support for insect cell culture. The Molecular and Cell Biology Core Facility (MCBCF), the Multi-Omics Core Facility (MOCF), and the Molecular Imaging Core Facility (MICF) of SLST provided technical support. The Analytical Chemistry platform of SIAIS provided technical assistance with protein identification by MS. This work was sponsored by grants from the National Key Research and Development Program of China, the National Natural Science Foundation of China and the ShanghaiTech University start-up fund.
Article link:
https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkab927/6406473
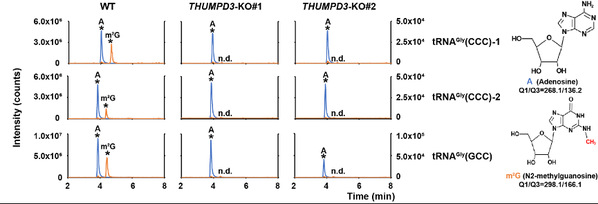
The level of m2G in tRNAGly(CCC)-1, tRNAGly(CCC)-2, and tRNAGly(GCC) became undetectable in both THUMPD3 knockout HEK293T cell lines

