Recently, two groups from the School of Life Science and Technology (SLST) at ShanghaiTech University, led by Prof. Wang Huayi and Prof. Lu Junxia, published a research article entitled “RIP3-mediated necroptosis is regulated by inter-filament assembly of RIP homotypic interaction motif” in Cell Death & Differentiation.
Necroptosis is a type of programmed necrosis induced by cytokines such as the tumour necrosis factor (TNF), which participates in the regulation of physiological and pathological processes including the immune response against pathogens and organ injury. Necroptosis is mediated by signalling complexes, called necrosomes, which contain receptor-interacting protein 3 (RIP3) and upstream effectors. In necrosomes, the RIP homotypic interaction motif (RHIM) of RIP3 and other effectors form an amyloidal complex. However, the mechanism by which the amyloidal necrosomes control RIP3 activation and cell necroptosis has not been determined.
The SLST researchers showed that RIP3 amyloid fibrils can further assemble into large fibrillar networks which present as cellular puncta during necroptosis. A viral RHIM containing necroptosis inhibitor M45 can form heteroamyloid with RIP3 in cells and prevent RIP3 puncta formation and cell necroptosis. Researchers have characterized mutual antagonism between RIP3-RHIM and M45-RHIM in necroptosis regulation, which is caused by distinct inter-filament interactions in RIP3, M45 amyloids as revealed with atomic force microscopy (AFM).
In this study, researchers proposed that the amyloidal formation is indispensable but not sufficient for RHIM mediated RIP3 kinase activation and cell necrosis. The necroptosis stimuli will induce cellular RIP3 to form amyloid fibrils and then self-assemble into large fibrillar networks through orderly inter-filament interactions. The latter step is critical and could be blocked either by specific mutations of RIP3 RHIM or by M45-RHIM amyloid incorporation.
Therefore, orderly inter-filament interactions are important in RIP3-mediated necroptosis. This study expands the realm of conceptual knowledge about programmed cell death and amyloid structure. Given the structural similarity of RIP RHIM and classical Aβ40, orderly inter-filament interactions of Aβ or other pathologic amyloids may contribute to amyloid deposition and disease progression.
This work was supported by the National Natural Science Foundation of China and the Start-up grant from ShanghaiTech University. The Molecular Imaging Core Facility, Molecular and Cell Biology Core Facility, Bio-logical Electron Microscope Microscopy Facility, and Analytical Instrumentations Center at ShanghaiTech University provided technical support.
Link:https://www.nature.com/articles/s41418-020-0598-9
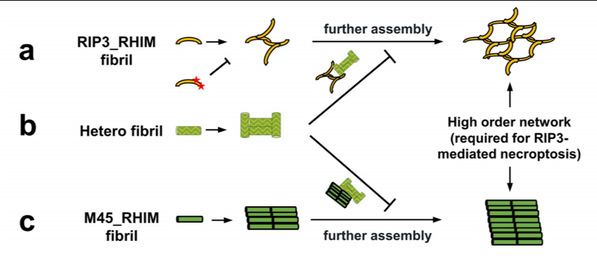
Figure: Schematic model of RHIM amyloid dependent RIP3 activation and M45 inhibition

