Recently, Research Associate Professors Liu Jia, from Lab of Macromolecular Drug Delivery and Ma Peixiang,from Lab of Phenotypic Screening at SIAIS, ShanghaiTech University, reported the first anti-CRISPR peptides. These peptides are derived from the periplasmic domain of the major coat protein G8P (G8PPD) of inoviridae bacteriophages and can allosterically inactivate CRISPR-Cas9. On 26 February 2020, this work has been published in Genome Biology, entitled “Allosteric inhibition of CRISPR/Cas9 by bacteriophage-derived peptides”.
As an emerging technology, CRISPR-Cas9 holds great potential in treating human diseases. Under clinical settings, CRISPR-Cas9 is often constitutively expressed in host cells. Prolonged expression of CRISPR-Cas9 can lead to elevated off-activity. Previously, anti-CRISPR proteins (Arcs) from the bacteriophage harbors and having the arms race nature of the bacterial CRISPR system have been discovered. It has been shown that these Acrs can improve the targeting specificity of CRISPR-Cas9 by the temporal control of Cas9 activity. In addition, a series of CRISPR-inhibiting small molecules have been identified using high throughput screen. Unlike the previously described small molecule and protein inhibitors of CRISPR, the inoviridae bacteriophage-derived CRISPR-inactivating peptides in this study can allosterically inhibit CRISPR-Cas9 and thus represents unique CRISPR-inhibiting agents.
In the present study, the researchers surprisingly discovered that intact M13 phage could inhibit the in vitro DNA cleavage activity of SpyCas9. Subsequent analyses identified the periplasmic domain of phage major coat protein G8P (G8PPD) as the source of CRISPR inhibition. In vitro cleavage assay showed that G8PPD can inhibit the apo form SpyCas9 without bound DNA or sgRNA, through an indirect competition with sgRNA. Further mass spectrometry and mutational analyses showed that the binding site of G8PPD was located in the PAM-interacting (PI) domain of SpyCas9, distal from the DNA and sgRNA binding sites. These results collectively suggest that G8PPD is an allosteric inhibitor of CRISPR-Cas9. Consistent with the in vitro experiments, pre-incubation of cells with G8PPD prior to Cas9/sgRNA transfection is necessary to achieve efficient inhibition of CRISPR-Cas9 activity in human cells. While Cas9/sgRNA and G8PPD are co-transfected, G8PPD can improve the specificity of CRISPR-Cas9 by the temporal control of SpyCas9 activity. This feature of G8PPD provides insights into developing next-generation CRISPR inhibitors for precision genome editing.
SIAIS is the primary affiliation for this study. Ph.D. candidate Cui Yanru and Mr. Wang Shaojie, engineer from the Lab of Macromolecule Drug Delivery are co-first authors. Research Associate Professors Liu Jia and Ma Peixiang are co-corresponding authors. This work is supported by the National Natural Science Foundation of China, the Lab of ADC Chemistry, Analytical Platform and High-Throughput Screening Platform at SIAIS of ShanghaiTech University.
Link to the paper:
https://genomebiology.biomedcentral.com/articles/10.1186/s13059-020-01956-x
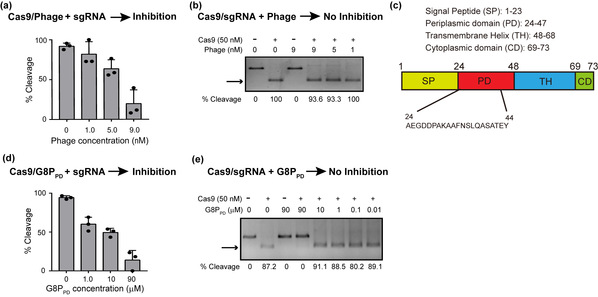
Fig. 1. Inhibition of the in vitro activity of SpyCas9 by intact M13 phage and phage-derived G8PPD peptides. a, Dose-dependent inhibition of SpyCas9 by intact M13 phage. b, Intact M13 phage does not inhibit the in vitro activity of assembled Cas9-sgRNA RNP. c, Structural organization of M13 phage major coat protein G8P. d, Dose-dependent inhibition of SpyCas9 by G8PPD. e, G8PPD does not inhibit the in vitro activity of assembled Cas9-sgRNA RNP.

