Researchers from the iHuman Institute at ShanghaiTech University, Chinese Academy of Sciences (Shanghai Institute of Materia Medica, SIMM), East China Normal University, University of Southern California, and the Danish biopharmaceutical company, Novo Nordisk, have determined the first “peptide-free inactive structure of the full-length human glucagon like peptide-1 receptor (GLP-1R) at atomic resolution. More than two years ago, the iHuman and SIMM collaboration team published the first inactive GLP-1R transmembrane domain crystal structure in Nature to understand what the transmembrane part of the receptor looks like before diabetes drug molecules bind. The new full-length structure reveals unique conformational state of the GLP-1R extracellular domain in the absence of a peptide ligand and deepens our understanding of activation mechanism of this receptor family following comparison with ligand-bound GLP-1R structures. The study entitled “Full-length human GLP-1 receptor structure without orthosteric ligands” was published online on March 9, 2020 at Nature Communications. Ms. Wu Fan, a Ph.D. candidate of iHuman Institute, ShanghaiTech is the first author, Drs. Raymond Stevens (iHuman Institute, ShanghaiTech University) and Song Gaojie (East China Normal University) are the co-corresponding authors.
GLP-1R is a class B G protein-coupled receptor (GPCR) that plays an important role in glucose homeostasis and one of the top diabetes drug targets. GLP-1 is the endogenous peptide of the receptor and displays a variety of physiological functions such as lowering blood glucose through enhanced glucose-dependent secretion of insulin, inhibiting glucagon secretion, suppressing food intake and slowing gastric emptying. Thus, GLP-1 peptide analogues have been successfully developed to treat type 2 diabetes and obesity such as ExenatideTM, LiraglutideTM, LixisenatideTM, and SemaglutideTM. Because of its prominence in metabolism regulations, its structural biology studies have attracted significant attention worldwide. A number of agonist-bound full-length GLP-1R structures have been reported in the past three years but that of the orthosteric ligand free receptor structure have eluded researchers until now.
“Unlike the traditional seven-transmembrane bundles of GPCRs, GLP-1R has a dynamic extracellular domain, a hallmark of class B GPCR family members. It is very challenging to solve a ‘peptide-free’ inactive structure because the two domains move dynamically relative to each other for receptor function, ” said Professor Song. “Through a productive collaboration with Novo Nordisk, we were able to use a non-competitive antibody to facilitate crystallization of the full-length peptide-free GLP-1R, ” said Ms. Wu. The structure reveals a unique closed conformation compared to previously solved agonist-bound GLP-1R structure, suggesting that huge conformational changes of the extracellular domain as well as the transmembrane domain are needed during the hormone peptide recognition and activation. Furthermore, results from negative stain electron microscopy (EM) and molecular dynamics (MD) simulations confirmed conformational dynamics of the extracellular domain during binding to GLP-1. “We proposed that the receptor is dynamic in the absence of a peptide and would favor a closed conformation stabilized by the weak interactions between the two domains, while peptide hormone will trigger the transition of GLP-1R from the closed to the otherwise energetically unfavourable open conformation,” said Professor Stevens.
“The newly disclosed structure points to a negative regulation function of the extracellular domain in class B GPCRs. This knowledge will certainly be helpful in the design of novel ligands for therapeutic use, ” commented Professor Wang Ming-Wei of SIMM.
Other authors include Professor Yang Linlin at Zhengzhou University, Lin Guangyao from ShanghaiTech University, Professor Jiang Hualiang from SIMM, Gye Won Hanfrom USC, Hang Kainiand Wu Lijie from iHuman Institute, ShanghaiTech University, Michael Hanson from GPCR consortium, and SteffenReedtz-Runge, Mette Laursen,Qiansheng Ren and Nikolaj Kulahin Roed from Novo Nordisk. The Cell expression, Cloning, Protein Purification and Functional Assay Cores of iHuman Institute provided technique support. The synchrotron radiation experiments were performed at the BL45XU of Spring-8, Japan. The EM data were collected at iNANO Cryo-Electron Microscopy Facility (EMBION). This work was supported by the GPCR Consortium, National Nature Science Foundation of China, the Ministry of Science and Technology of China, Shanghai Municipal Government and ShanghaiTech University.
The link of the paper: https://www.nature.com/articles/s41467-020-14934-5
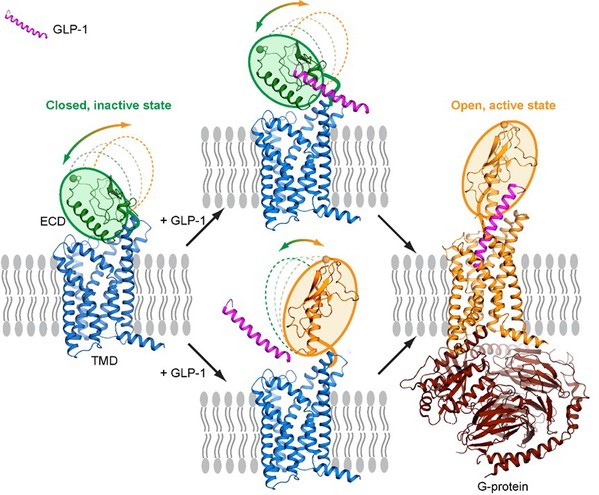
Fig. 1 The canonical two-domain activation pathway

Fig. 2 Wu Fan, a Ph.D. candidate of iHuman Institute, ShanghaiTech is the first author

