Recently, a collaborative study led by Assistant Professor Zhao Suwen and Assistant Professor Zhong Guisheng at iHuman Institute and School of Life Science and Technology, and Research Associate Professor Wu Dong at iHuman Institute reported two crystal structures of human α2A adrenergic receptor (α2AAR) in complex with a partial agonist and an antagonist, and discovered key non-conserved residues which play a key role in partial agonism and biased signalling. The three corresponding authors reported the crystal structure of human α2C adrenergic receptor (α2CAR) and the molecular basis of its subtype selectivity as well. Both studies, “Structural basis of the diversity of adrenergic receptors” and “Molecular mechanism for ligand recognition and subtype selectivity of α2C adrenergic receptor” were published simultaneously back-to-back in Cell Reports (on-line Dec 3, 2019 ).
There are nine human adrenergic receptors (α1A, α1B, α1D, α2A, α2B, α2C, β1, β2 and β3) that mediate the central and peripheral actions of catecholamines. Adrenergic receptors couple to different G-proteins, with α1, α2 and β types mainly coupling to Gq, Gi, and Gs, respectively. Exceptionally, α2AAR has a dual pharmacological effect in that it simultaneously couples to Gi and Gs to inhibit or stimulate adenylyl cyclase activity. At low agonist concentrations, α2AAR mainly couples to Gi, while at high concentrations, Gs coupling dominates. This unusual dual effect has not been well explained. Partial agonists of α2AAR, such as clonidine and dexmedetomidine, tend to have more therapeutic benefits than full agonists. The action mechanism of these partial agonists of α2AAR remains incompletely understood.
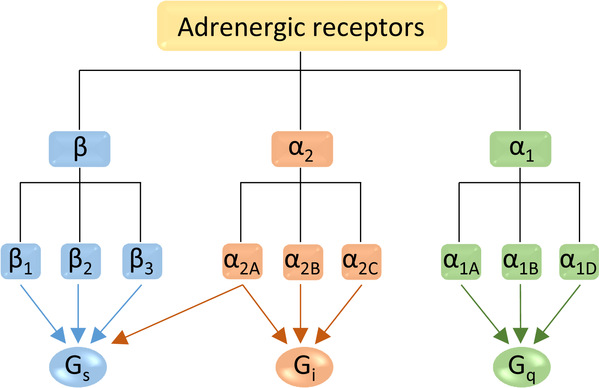
Figure 1. Subtypes of adrenergic receptors and their downstream G protein pathways.
Here, by determining two crystal structures of α2AAR in complex with a partial agonist and an antagonist, the team discovered key residues from the ligand binding pocket (Phe7.39 and Tyr6.55) to G protein coupling region (Ile34.51 and Lys34.56) that play a key role for the interplay between partial agonism and biased signaling of α2AAR. This study provides insight into the diversity of ligand binding and G-protein coupling preference of adrenergic receptors, and lays the foundation for the discovery of next generation drugs targeting these receptors.
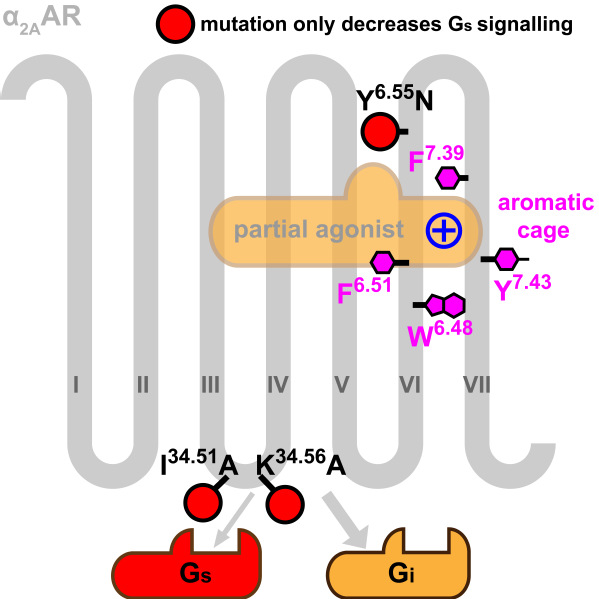
Figure 2. Ligand binding pocket (magenta residues) and key residues (red balls) for Gi biased pathway in α2A adrenergic receptor.
As presynaptic inhibitory receptors, α2AAR and α2CAR both regulate the release of norepinephrine but at high and low stimulation frequencies, respectively. Although the three α2AR subtypes differ considerably in their tissue distribution and physiological roles, they are highly homologous in the trans-membrane region and thus share most agonists and antagonists, limiting receptor-specific research to genetic modification in animals and in vitro investigations in cell lines.
To this end, we solved the crystal structure of human α2CAR in complex with RS79948, a non-selective antagonist of α2ARs. Based on the solved α2AAR and α2CAR structures and supported by mutagenesis, modelling and functional assays, we are able to provide key insight into the subtype selectivity of α2ARs. Our results indicate that an α2CAR specific D206ECL2-R409ECL3-Y4056.58 network and a different interaction network in α2AAR formed by Y98ECL1, R187ECL2, E189ECL2, and R4057.32 which acts as a lid covering the entrance of the ligand binding pocket, are involved in α2 adrenergic subtype selectivity. This study provides molecular foundation for α2CAR selective ligands beneficial to in vivo subtype functional study and new insights to understanding GPCR subtype selectivity.
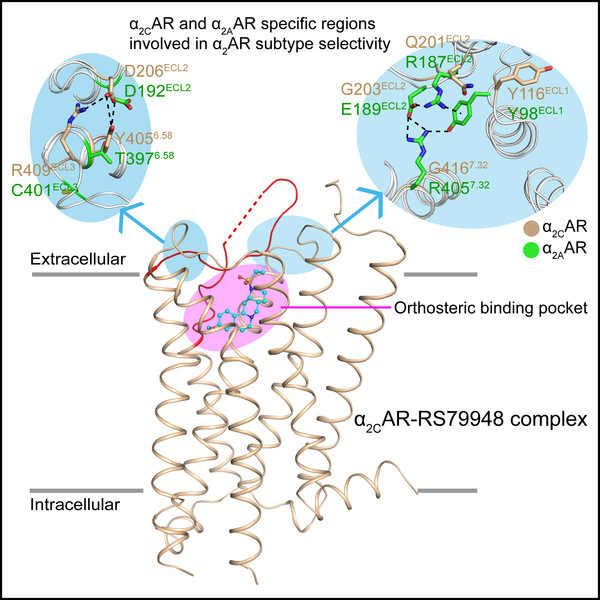
Figure 3. The structure of human α2CAR-RS79948 complex and the specific extracellular regions in α2CAR and α2AAR involved in α2AR subtype selectivity.
Graduate student Qu Lu, co-mentored by Professors Liu Zhijie and Raymond Stevens, and Research Assistant Professor Zhou Qingtong are joint first authors of “Structural basis of the diversity of adrenergic receptors.” Graduate student Chen Xiaoyu and the director of Assay Development Core Xu Yueming are joint first authors of “Molecular mechanism for ligand recognition and subtype selectivity of α2C adrenergic receptor.” Tao Houchao’s lab and iHuman cores also made their contributions to this work.
Read more at:
Structural basis of the diversity of adrenergic receptors: https://www.cell.com/cell-reports/fulltext/S2211-1247(19)31410-X
Molecular mechanism for ligand recognition and subtype selectivity of α2C adrenergic receptor:https://www.cell.com/cell-reports/fulltext/S2211-1247(19)31445-7

