On August 19th, an article by a research group led by iHuman Institute and SLST Assistant Professor Zhong Guisheng, working with collaborators Southeast University Professor Chai Renjie and Fudan University Professor Li Huawei was published in Nature Communication, entitled ‘AAV-ie enables safe and efficient gene transfer to inner ear cells.’ They have identified a novel adeno-associated virus (AAV) vector, AAV-ie, which can transduce the cochlear supporting cells (SCs) with high efficiency, representing a vast improvement over conventional AAV serotypes. Furthermore, after AAV-ie-mediated transfer of the Atoh1 gene, many SCs trans-differentiated into new HCs. These results suggest that AAV-ie is a useful tool for cochlear gene therapy and for investigating the mechanism of HC regeneration.
Hearing loss, one of the most common sensory disorders, affects over 6.8% of the world’s population (approximately 500 million people). Recently, AAV mediated gene therapy has emerged as a possible and promising strategy for the treatment of hearing loss.
Both hair cells (HCs) and supporting cells (SCs) in the cochlea can be targeted for AAV mediated gene therapy. Half of the cases of sensorineural hearing loss are due to genetic mutations in HCs and SCs. Some key deafness genes mainly express and have functions in the SCs, such as GJB2. Moreover, recent studies have shown that SCs are promising inner ear progenitors from which HCs can be regenerated. Thus, SCs are important potential targets for gene therapy, not only for correcting genetic hearing defects but also for HC regeneration. However, conventional AAV serotypes have been evaluated in the cochlea and all those tested showed low transduction ratio in SCs.
Here, Zhong Guisheng’s group designs an AAV variant named AAV-ie, for gene delivery in the mouse cochlea. Ex vivo transduction of mouse organotypic explants shows that AAV-ie can infect nearly 90% of the SCs. In vivo experiments show that after RWM injection of these AAVs into the cochlea, AAV-ie transduces SCs with significantly higher efficiency than other AAV serotypes (Fig. 1).
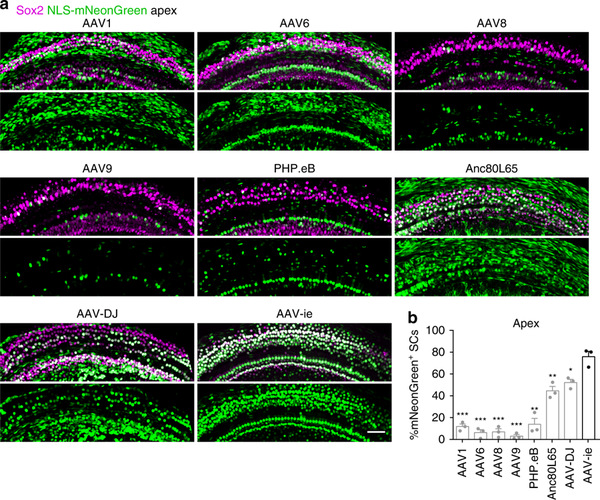
Fig. 1 AAV-ie infects cochlear supporting cells with high efficiency
Round window membrane (RWM) injection of AAV-ie is safe, as indicated by well-preserved HCs and hearing function. Further, after delivering Atoh1 gene into mouse cochlea by AAV-ie, many new HCs are generated, indicating the potential of the AAV-ie vector for HC regeneration (Fig. 2).
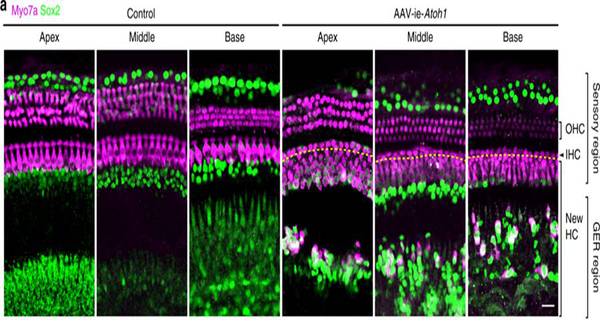
Fig. 2 AAV-ie-Atoh1 induces new hair cells (HCs) in vivo
Dr. Tan Fangzhi, Dr. Chu Cenfeng, Dr. Qi Jieyu and Dr. Li Wenyan are co-first authors. Professor Zhong Guisheng, Professor Chai Renjie, Professor Li Huawei and Dr. Tan Fangzhi are the co-corresponding authors.
Read more at: https://www.nature.com/articles/s41467-019-11687-8

