A research collaboration between the laboratories of Yang Guang and Richard Lerner of SIAIS in ShanghaiTech University and the laboratory of Xu Tianle in Shanghai Jiao Tong University School of Medicine has led to an article published in the Proceedings of the National Academy of Sciences of the United States of America on July 25th. The article, “Selection of an ASIC1a-blocking combinatorial antibody that protects cells from ischemic death” lists Dr. Qiang Min and graduate student Dong Xue as co-first authors and Richard Lerner, Yang Guang and Qu Zhihu as co-corresponding authors.
Acid-sensing ion channels (ASICs) are voltage-insensitive cation channels widely distributed in central and peripheral nervous systems throughout the body. In the nervous system, ASIC1a is involved in the synaptic plasticity, learning and memory, pain sensation and ischemic stroke conditions, and it represents a novel therapeutic target in stroke and pain, etc. Despite their high potency, small molecules and animal venom peptides inhibiting ASIC1a are not selective or biologically unstable in humans. Monoclonal antibodies could represent novel ASIC1a modulators with greater potency, subtype selectivity, and biological stability. By panning from a combinatorial human antibody library, researchers from Yang and Lerner’s lab obtained a highly specific antibody targeting the human ASIC1a (hASIC1a) which could become a new drug for stroke and other neurological diseases.
To identify functional anti-hASIC1a antibodies, the hASIC1a was purified and assembled in the nanodiscs such that the target channel has a configuration as close as possible to its natural state in the plasma membrane. “Differential selection” was applied in the iterative selection process and a highly potent and selective antibody ASC06 (KD = 7.9 nM) targeting only the ASIC1a subtype was identified. The specificity of ASC06 was further demonstrated in the primary neurons from the ASIC1a knockout mice. Images from the transmission electron microscope clearly shows that in each of the ASIC1a channel consisting of three subunits, up to three ASC06 antibodies can bind to the interfaces between two subunits and form a stable complex. Molecular dynamics (MD) was used to reconstitute the 3D structure of the complex (Figure 1).
Functional studies revealed that ASC06-IgG1 is able to inhibit acid-induced opening of the ASIC1a channel in a dose-dependent way. Moreover, it blocks the transport of cations including calcium influx (IC50 = 2.8 nM). As a proof-of-concept in vivo, ASC06-IgG1, administrated three hours after ischemia, showed an effective protection of the brain cells from death in MCAO mice (Attachment Figure 2). Patch-clamp studies further demonstrated that, unlike the PcTx1 peptide, which binds to the acidic pocket of ASIC1a and induced an alkaline-shift in both the activation and the steady-state desensitization (SSD) curves of pH, ASC06-IgG1 inhibited the channel in a non-competitive manner.
Drs. Zha Zhaoli, Francesco Zonta, Zhao Lixia and Damiano Burrato as well as SIAIS graduate students Yuan Chao and Tao Dongping; and Shanghai Jiao Tong University School of Medicine graduate students Zuo Xiaokun, Song Xinglei and Huang Chen contributed to the study. Many research teams such as Dr. Li Guangwei’s team and Dr. Hu Qin’s team at Shanghai Jiao Tong University School of Medicine, Dr. Nie Yan’s team and Dr. Yang Bei’s team at SIAIS provided constructive and intellectual input and support. In addition the platforms of Protein & Gene, Informatics, Cell sorting, Imaging, and HTS from SIAIS and Electron Microscopy System at National Facility for Protein Science in Shanghai (NFPS), Zhangjiang Lab provided technical support.
Read more at:http://www.pnas.org/content/early/2018/07/23/1807233115
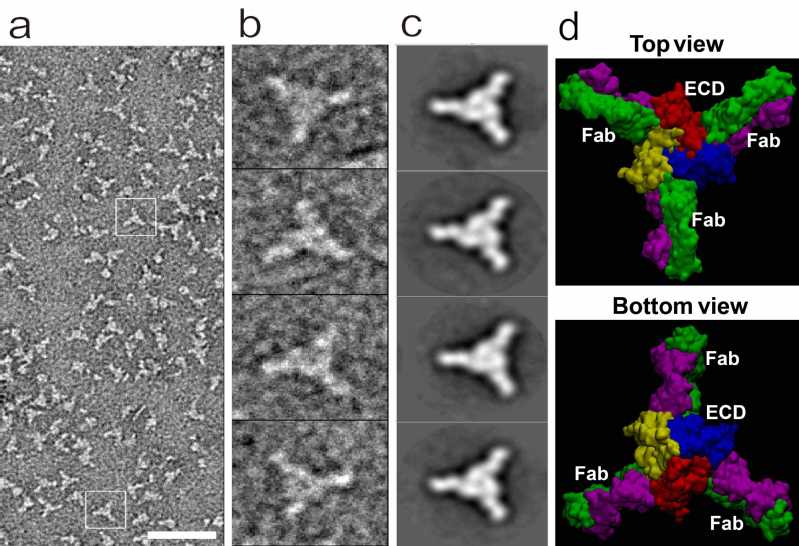 Negative-staining EM analysis of the hASIC1a & ASCO6 complex, with molecular dynamics (MD) simulation of the complex
Negative-staining EM analysis of the hASIC1a & ASCO6 complex, with molecular dynamics (MD) simulation of the complex
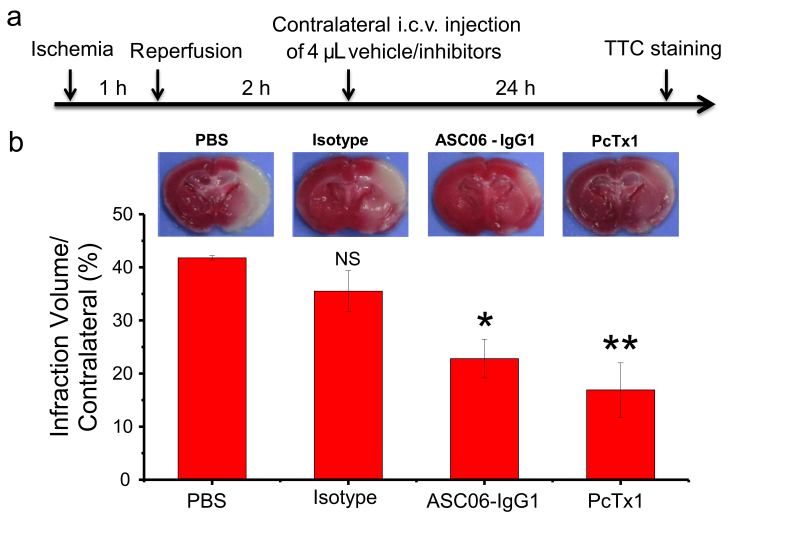
Neuroprotective effect of the ASIC1a specific antibody on the ischemic mice model

