Recently, Dr. Ji Quangjiang’s group from the School of Physical Science and Technology deciphered the molecular mechanism of how the pathogen Staphylococcus aureus utilizes staphylopine (StP) to recognize and capture metal ions from its surroundings.
Their research was published in an article in Proceedings of the National Academy of Sciences titled “Mechanistic insights into staphylopine-mediated metal acquisition.”
Transition metals are required trace elements for all forms of life. More than 30% of all proteins contain a transition metal cofactor. Metals serve as catalytic centers for enzymatic reactions, contribute to overall protein stability, or act as signaling agents. Transition metals are essential for microbial survival and pathogenesis, while pathogens must obtain nutrient metals from the host to colonize and cause disease.S. aureus is a Gram-positive bacterium that is the leading cause of hospital- and community-acquired infections. This pathogen causes a wide variety of infections, ranging from minor skin infections to life-threatening diseases, such as septicemia, necrotizing pneumonia and toxic shock syndrome. Research on how S. aureus acquires metal ions from the human host and causes disease is crucial for the development of strategies to prevent and treat S.aureus infections. S. aureus biosynthesizes StP, a broad-spectrum metallophore that binds, mediates the transport of transition metals such as Ni2+, Zn2+, Co2+ and contributes to staphylococcal virulence. (Science, 2016, 352, 1105). However, the molecular mechanism of how StP/metal complexes are recognized and transported remained unclear.
Ji Lab employed isothermal titration calorimetry (ITC) to examine the interactions between StP/metal and the Cnt solute-binding protein (CntA) and discovered that StP/metal recognition by CntA is selective and specific while both the metallophore StP and metals are indispensable for CntA recognition. Then they obtained multiple structures of CntA in apo form and in complex with StP and three different metals ( Ni2+、Zn2+、Co2+ ) (Fig. A) and determined that StP/metal binding triggers a notable interdomain conformational change in CntA. They also found the ten key residues (Y27, W103, R140, R225, R393, Q404, W406, Y410, N423, and Y497) in the ligand-binding pocket, deciphering the detailed interactions between CntA and StP/metal (Fig. B/C). Furthermore, Ji group used the recently developed CRISPR/Cas9-based genome editing tool pCasSA and quickly created five independent single amino-acid mutations in the S. aureus genome, allowing systematic investigations of the role of StP/metal recognition invivo. Taken together, their work unveils the detailed StP-recognition mechanisms by structural characterizations of the CntA/StP/metal complexes as well as the ligand-free CntA. These discoveries shed light into the explorations of novel metal-acquisition mechanisms in other microbes and suggest that targeting StP/metal recognition could be a feasible strategy to counter bacterial infections.
The first author of the paperis Song Liqiang, postdoc assistant from the School of Physical Science and Technology, and the second author is graduate student Zhang Yifei, while Ji Quanjiang is the corresponding author. Their research was supported by the National Key R&D Program of China, the National Natural Science Foundation of China.
Read more: http://www.pnas.org/content/early/2018/03/21/1718382115
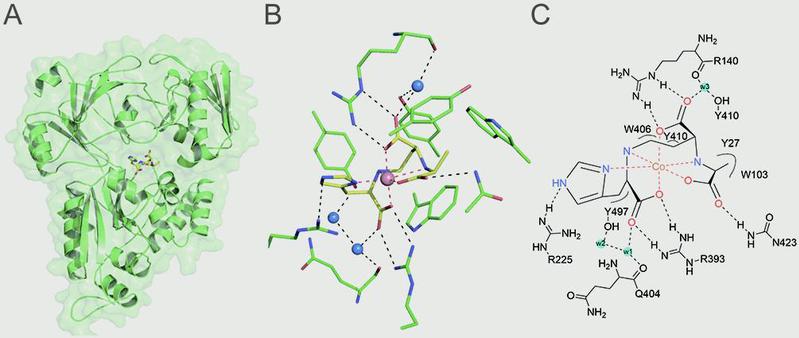 (A) The surface structure of the CntA/StP/Co2+ complex. (B/C) Detailed interactions between CntA and StP/Co2+.
(A) The surface structure of the CntA/StP/Co2+ complex. (B/C) Detailed interactions between CntA and StP/Co2+.

