Post-translational modification (PTM) plays an important role inregulating the physiological activity of proteins, controlling the enzymecatalytic activity and protein-protein recognition in the biological process. The chemical modification of proteins can study the physiological mechanism of proteins by mimicking PTM process, as well as tracking proteins invitro/vivoand developing therapeutic protein conjugates, particularly antibody drug conjugates. Recently, School of Physical Science and Technology (SPST) Assistant Professor of Materials Biology Dr. Yang Xiaoyu and his collaborators made a breakthrough in selective methionine bioconjugation. Their paper, "Redox-based reagents for chemoselectivemethionine bioconjugation,” was published in Science on February 10th.The research was conducted with Professor Christopher J. Chang and Professor F. Dean Toste of the University of California-Berkeley Department of Chemistry. Dr. Yang was co-first author of the paper and ShanghaiTech University is one of the affiliations.
Dr. Yang and his collaborators developed a novel chemical labeling strategy, termed Redox Activated Chemical Tagging (ReACT), which for the first time enables chemoselective methionine bioconjugation in proteins and proteomes. In their work, they reported using oxaziridine-based reagents toachieve highly selective, rapid, and robust methionine labeling under a rangeof biocompatible reaction conditions. Dr. Yang and the research team alsohighlighted the broad utility of this conjugation method to enable precise additions of payloads to proteins, synthesis of antibody-drug conjugates, and identification of hyper-reactive methionine residues in whole proteomes.
This breakthrough protein bioconjugation technology has great potential in the field of life science research and biomedicine development. In life science study, it can mimic the post-translational modification (PTM) innature, which is used to study the mechanism of protein physiological function. In the field of biomedicine development, it can be used to develop therapeuticprotein conjugates, especially antibody drug conjugates (ADCs). Antibody drugconjugates (ADCs) are a new class of therapeutic agents, and are gaining increasing attention from pharmaceutical companies. ADCs are constituted bymonoclonal antibodies and potent toxic drugs through the biologically active linker, serving as efficiently targeted anti-cancer drugs. Because of its accurate identification of targeted and non-cancer cells, it can greatly reduce toxic side effects and improve efficacy. A report released by Research & Markets, a well-known global market research company in 2014, shows that the ADC market will experience rapid growth over the next 10 years and is expected to reach $10 billion by 2024.
Read more at:http://science.sciencemag.org/content/355/6325/597
and: http://cen.acs.org/articles/95/i7/Modifying-methionine-proteins.html
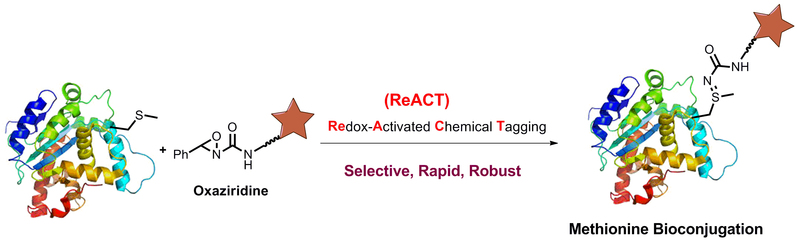
Selective Methionine Bioconjugation
- About
- News
- Faculty
- Research
-
Academics
- School of Physical Science and Technology (SPST)
- School of Life Science and Technology (SLST)
- School of Information Science and Technology (SIST)
- School of Entrepreneurship and Management (SEM)
- School of Creativity and Art (SCA)
- Institute of Humanities (IH)
- School of Biomedical Engineering (BME)
- Shanghai Institute for Advanced Immunochemical Studies (SIAIS)
- iHuman Institute
- Institute of Mathematical Sciences (IMS)
- Center for Transformative Science (CTS)
- Institute of Carbon Neutrality (ICN)
- Shanghai Clinical Research and Trial Center
- Tech Transfer
- Global
- Campus Life

