Recently, Assistant Professor Liu Jia’s group at SIAIS along with his collaborator, Professor Qu Jieming at Ruijin Hospital and the Institutes of Respiratory Diseases of Shanghai Jiao Tong University designed a novel surfaceome CRISPR library and identified OLFML3 as a novel host factor of rhinovirus (RV). They found that OLFML3 could promote RV infection by antagonizing type I interferon (IFN) signaling in a suppressor of cytokine signaling 3 (SOCS3)-dependent manneron October 22 in an article entitled “Surfaceome CRISPR screen identifies OLFML3 as a rhinovirus-inducible IFN antagonist”.
RV is known as the prevalent pathogen causing common cold and has also been associated with other severe respiratory symptoms including asthma exacerbations and chronic obstructive pulmonary disease. The diverse categories of RV make the task of dissecting host-pathogen interactions very sophisticated. High-throughput functional genomics approaches can be employed for dissection of host-pathogen interactions. Particularly, in recent years, CRISPR-based functional genomics have been widely used and facilitate the identification of emerging and important cellular host factors. However, conventional CRISPR screens are mainly based on genome-wide approach, which are prone to false positive hits creating a background noise.
In the present study, the researchers constructed a novel surfaceome CRISPR library, and systematically compared surfaceome and genome-wide CRISPR libraries. They found that the surfaceome CRISPR library has higher efficiency in identifying RV host factors that have important biological functions.
In addition, the researchers identified OLFML3 as a novel host factor of RV and proved that OLFML3 has inducible expression upon RV infection and could antagonize the innate immune response of host cells. By in-depth study of the mechanism of OLFML3 action, they found that OLFML3 inhibits type I IFN signaling and promotes RV infection by a SOCS3-dependent inhibition of IFNs and IFN-stimulated genes (ISGs). Notably, the biological functions of OLFML3 are poorly understood and this study is the first report describing the immunosuppressive function of OLFML3 during pathogen immunity.
Postdoctoral scholar Mei Hong of Liu Jia’s Lab, Research Assistant Professor Zha Zhao and Engineer Wang Wei of the Biomedical Big Data Platform at SIAIS are the co-first authors. Prof. Liu Jia and Prof. Qu Jieming are the co-corresponding authors. ShanghaiTech University is the primary affiliation for this study.
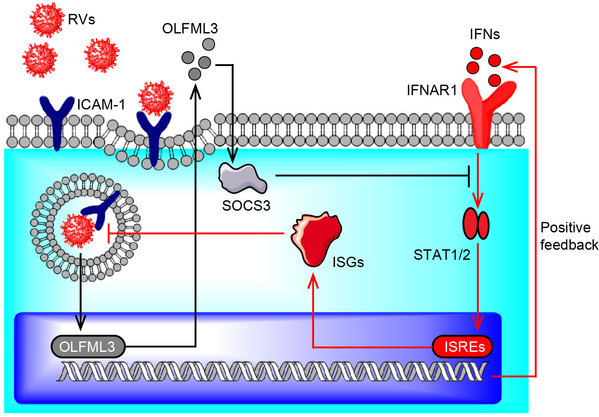
Schematic diagram illustrating OLFML3 and SOCS3-mediated inhibition of type I IFN signaling during RV infection
Link to the paper:
https://genomebiology.biomedcentral.com/articles/10.1186/s13059-021-02513-w

