Cancer is one of the biggest killers threatening human life and chemotherapy, which kills cancer cells with molecular drugs that are toxic to cells, is one of the main ways to treat cancer. For metastatic tumors, chemotherapy is the most effective method. However, chemotherapy drugs can also kill a large number of normal cells, causing severe toxic side effects and seriously damaging the physical and mental health of patients. The question of how to ensure that chemotherapy drugs can kill cancer cells specifically has long been pursued actively.
Human and other aerobic organisms cannot survive without oxygen, but cells contain a large number of physiological functional sites that are easily destroyed by oxidative environment. Upon development of a new methodology for mechanistic medicinal chemistry, a team led by Assistant Professor Lin Bolin of School of Physical Science and Technology recently discovered for the first time an unknown defect in the reductive protection mechanism ubiquitous in cells of aerobic organisms and also unveiled for the first time how classical alcohol-abuse drug disulfiram leverages this defect in its broad-spectrum clinical cancer treatments. The discovery provides a new general principle that may lead to chemotherapeutic drugs and methods to kill various cancer cells specifically. The work was published in Angewandte Chemie International Edition under the title Universal Anticancer Cu(DTC)2 Discriminates Between Thiols and Zinc(II) Thiolates Oxidatively.
Disulfiram has been used for almost 70 years as a drug for alcohol abuse. It produces acute ethanol sensitivity by preventing the body from breaking down acetaldehyde, including immediate and enhanced hangover effects. But two years ago, in an epidemiological study, scientists found that Cu(DTC)2 (DTC: diethyldithiocarbamate), a metabolite of disulfiram in the presence of Cu(II) in vivo, had a clinical treating effect on almost all cancers. Although biological evidence has been collected to show that Cu(DTC)2 targets the Zn-thiolate site of NPL4 protein to kill cancer cells. The molecular-level chemical mechanism for how such a copper(II) complex interacts with the reductive intracellular environment to target the zinc-thiolate site remains unclear.
Zinc and copper are essential trace elements for life. Many important enzymes and proteins in mammals have zinc-thiolate sites in their core functional units. In particular, about 1% of human gene is coding sequences of zinc finger proteins featured with zinc-thiolate sites, which play a key role in many physiological processes. However, zinc-thiolate sites are easily destroyed by oxidants such as bivalent copper ions. Aerobic organisms have evolved a reductive protection mechanism based on high-concentration intracellular thiols and NAD(P)H. Such a mechanism provides a tightly-regulatory reductive protection for key physiological function sites vulnerable to oxidative damage, such as zinc-thiolate sites, through reductive quenching of oxidants entering the cell. In layman terms, the mechanism functions like a firewall that protects computer operating systems from viruses. It is typically believed that bivalent copper ions enter cells and are reduced to univalent copper ions by thiols/NAD(P)H, thus losing their inherent reactivity to oxidatively damage Zn-thiolate sites.
Considering the structural complexity of zinc(II)-thiolate enzymes/proteins and the lack of characteristic spectroscopic features to monitor chemical changes of the zinc(II) sites, probing clean bioinorganic model reactions of synthetic zinc(II) complexes with well-defined structures mimicking those in living organisms is an effective way to obtain molecular-level insights into their core mechanisms. Surprisingly, Lin’s team found that among all studied ligands of copper(II), DTC displays a unique redox-tuning ability that enables copper(II) to resist the reduction by thiols while retaining its ability to oxidize zinc(II) thiolates to form disulfides. This work proves for the first time that it’s possible to develop oxidants to discriminate between thiols/NAD(P)H and zinc(II) thiolates, alluding to a new chemical principle for how oxidants, especially universal anticancer Cu(DTC)2, might circumvent the intracellular reductive defense around certain zinc(II)-thiolate sites of proteins to kill malignant cells.
Based on the findings of this work and literature reports, Lin's team proposed the first possible molecular-level mechanism for the universal anticancer activities of disulfiram. First, tumor tissues selectively accumulate copper ions; there is considerable literature to support this. Next, DTC is generated from regular metabolism of disulfiram in vivo. After encountering copper ions accumulated tumor tissues in the inner circulation, Cu(DTC)2 is formed in situ due to the formation of strong chelating covalent coordination bonds, allowing Cu(II) to avoid being trapped by the regulatory proteins. Direct penetration through the cell membrane then occurs due to the strong hydrophobicity and the small size of Cu(DTC)2. Subsequently, Cu(DTC)2, due to its special oxidative selectivity, overcomes the intracellular reductive protective barrier, and then induces protein denaturation by oxidizing the zinc-thiolate sites in the zinc finger domain of NPL4 or other proteins. Therefore, pathways, including p97-ufd1-npl4 that degrades ubiquitinated proteins, are inhibited and cancer cells are selectively killed.
This work clearly reveals the first possible molecular-level mechanism underlying the universal anticancer activities of disulfiram and opens up a new general mechanism for potential chemotherapeutic drugs and methods to kill various cancer cells specifically. The reviewer of the paper highly praised this work as ground-breaking.
Graduate student Xu Luyan and undergraduate student Xu Jialin are the co-first authors. This work was supported by a grant from the National Natural Science Foundation of China.
Read more at: https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.201814519

Figure 1. The unknown anticancer mechanism of disulfiram
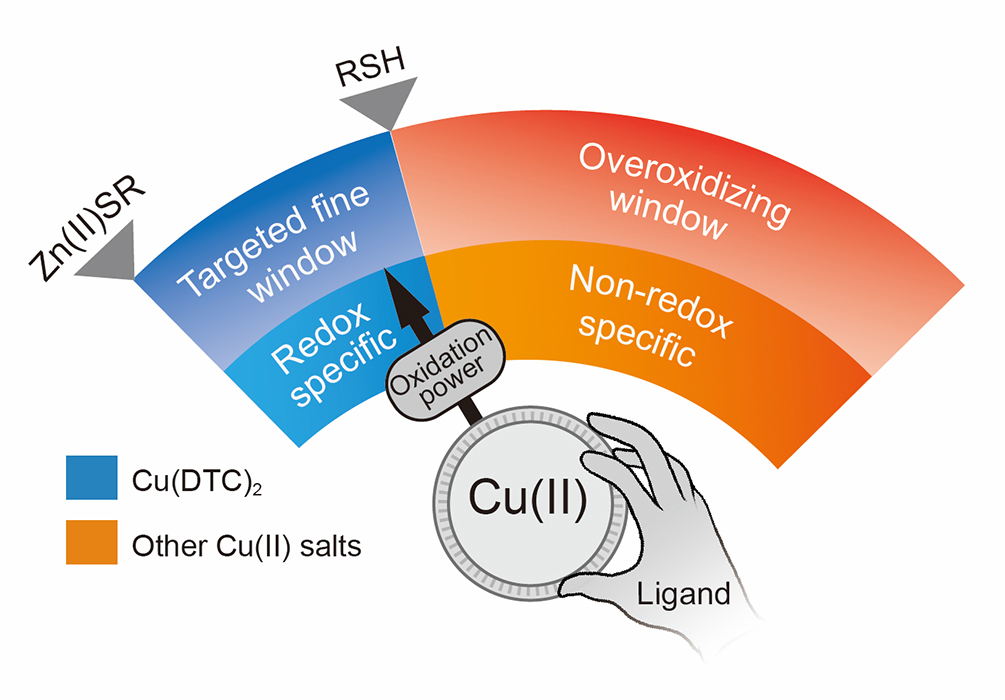
Figure 2. Tuning of the oxidative power of Cu(II) into the fine reduction window between thiols and Zn(II) thiolates by ligands.

