Methamphetamine (METH), commonly known as crystal meth, is one of the most abused substances, posing a serious burden on human health and society. Currently, there are no approved drugs available for the treatment of METH addiction. METH addiction primarily operates by modulating dopamine release and reuptake, a widely known mechanism. Recent studies indicated that METH can directly bind to Trace Amine-Associated Receptor 1 (TAAR1), a trace amine receptor, activating the downstream G-protein signaling pathway. This interaction is suggested to be a critical node in METH signaling transduction within the body. Additionally, TAAR1 is believed to play a crucial role in regulating the neurobiological effects of METH and other amine molecules, such as endogenous phenethylamine, as well as clinical drug candidates for conditions like schizophrenia. Therefore, a thorough investigation of the interaction mechanisms between METH and other amine molecules with TAAR1 through structural biology may provide significant insight for drug addiction treatment and the development of novel antipsychotic drugs.
On November 7, the ShanghaiTech research team published their latest results in Nature in the article entitled “Recognition of methamphetamine and other amines by trace amine receptor TAAR1” in the form of “Accelerated Article Preview (AAP)”.
TAAR1 is a G-protein-coupled receptor primarily distributed in the brain, capable of recognizing various biogenic amine substances, including endogenous substance β-phenethylamine (β-PEA), and participates in neurotransmitter regulation. Unlike other eight trace amine receptors in the TAAR family, TAAR1 is mainly distributed in the monoaminergic nuclei of the brain and their projection areas, where dopamine neurons and serotonin neurons are located. This distribution aligns with TAAR1’s critical role in regulating reward, cognition, and emotions. Consequently, TAAR1’s unique physiological function is closely associated with various mental disorders. Numerous studies have suggested that TAAR1 agonists have the potential for treating schizophrenia, depression, and drug addiction.
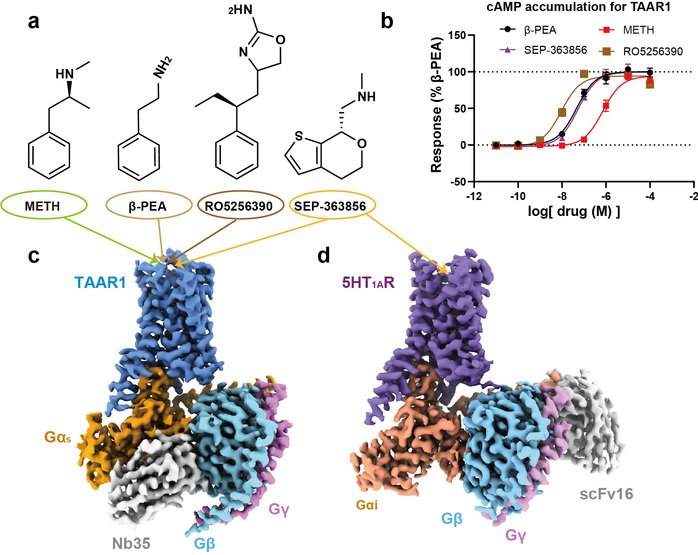
In this study, researchers employed the cryo-electron microscopy technology to determine, for the first time, the high-resolution structures of the human TAAR1-Gs protein complex with METH, endogenous ligand β-PEA, selective agonist RO5256390 and clinical-stage candidate drug SEP-363856 (Figure 1). Structural analysis revealed that METH is positioned in the orthosteric binding pocket of TAAR1, specifically interacting with TAAR1 through polar interactions with aspartic acid at position 103 and tyrosine at position 294 (Figure 2a-b). Furthermore, a hydrogen bond network formed by the ligand-binding pocket and surrounding amino acids contributes to stabilizing the interaction between the ligand and TAAR1 (Figure 2b-c). The second extracellular loop of TAAR1 forms a unique “lid” structure, involving the highly specific phenylalanine at position 186 and other hydrophobic amino acids, directly or indirectly participating in ligand interaction, thus establishing TAAR1’s distinctive molecular recognition mechanism (Figure 2a). Compared to the endogenous ligand β-PEA, METH exhibits weaker polar interactions with the key residues aspartic acid at position 103 and serine at position 107, resulting in decreased affinity to TAAR1. This may serve as the structural basis for β-PEA’s more efficient binding to TAAR1 than METH (Figure 2d). Molecular dynamics simulations and systematic mutation and functional experiments validated these conclusions.
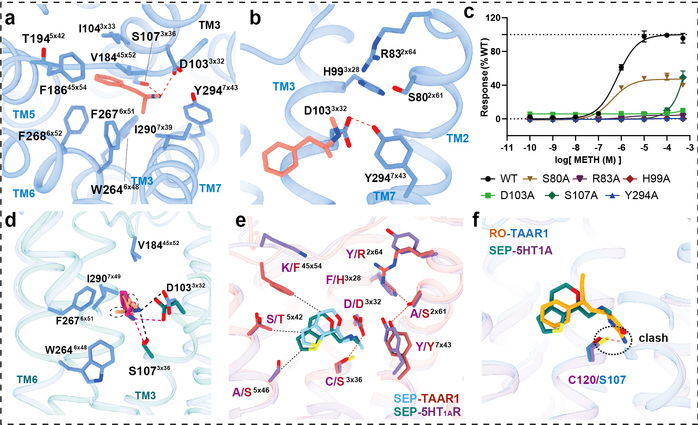
Figure 1: Functional characterization of the four TAAR1 agonists presented in this study and overall structures for the four agonist-bound TAAR1-Gs complex as well as one 5-HT1AR complex.
Additionally, this study also elucidated the complex structures of other drugs with clinical research potential that activate TAAR1, such as the dual-target amine compound SEP-363856 (clinical candidate drug, also known as Ulotaront) and TAAR1-selective agonist RO5256390, revealing the molecular mechanisms behind their polypharmacology and selectivity (Figure 1). SEP-363856 exhibits agonist activity for both TAAR1 and 5-HT1A receptors, with higher activation efficacy for TAAR1. Structural alignment analysis found that although there is some similarity in their binding pockets, TAAR1-specific polar interaction networks contribute to enhancing SEP-363856’s affinity (Figure 2e). The high affinity and high selectivity of RO5256390 for TAAR1 stem from additional interactions formed by its 2-aminotetrazole group with amino acid residues in the binding pocket, while causing steric hindrance in the binding pocket of the 5-HT1A receptor (Figure 2f). These findings provide a theoretical foundation for the precise design of new drugs.
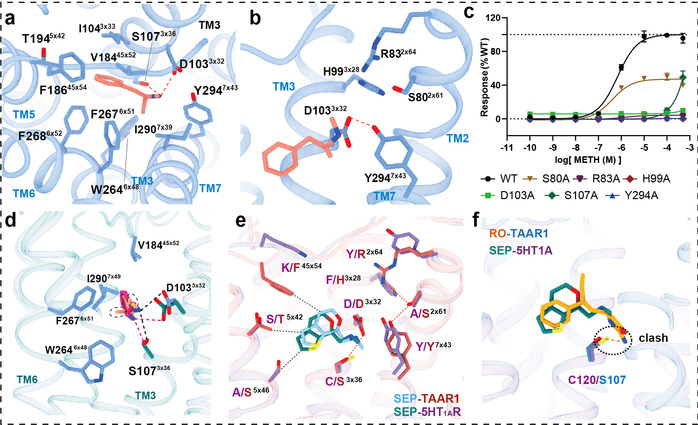
Figure 2: Structural analysis of the agonist-bound TAAR1-Gs complex structures for elucidation of the ligand recognition mechanism.
This research achievement is a milestone in the field of METH research, likely opening up a new area of biogenic amine research beyond classical neurotransmitters such as serotonin and dopamine. The study systematically reveals the key structural elements of the interaction between METH and other amine compounds with TAAR1, providing a solid foundation for the development of new drugs for treating drug addiction and neuropsychiatric diseases. Simultaneously, as TAAR1 is the last unrevealed receptor structure in the monoaminergic system, this study has filled a gap in the field of amine receptor structures, holding significant implications for understanding the pharmacology of the monoaminergic system and potentially driving the development of a new generation of safer and more efficient drugs.
Other than Prof. Xu Fei, three collaborators from other institutes were also the co-corresponding authors. They are Professor Xu Huaqiang from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Professor Wang Sheng from the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, and Dr. Liu Wenbin from the Shanghai Research Institute of Criminal Science and Technology. Zheng You, an SLST student of the Class of 2023 was one of the co-first authors.
Full article link: https://www.nature.com/articles/s41586-023-06775-1
**This article is provided by Prof. Xu Fei

