Recently, a research article entitled “LUBAC and OTULIN regulate autophagy initiation and maturation by mediating the linear ubiquitination and the stabilization of ATG13” was published online in Autophagy. This work was done by Professor Liu Yanfen’s group from the School of Life Science and Technology (SLST) at ShanghaiTech University.
Autophagy is a self-eating system that is essential for cellular homeostasis. Dysregulation of autophagy is linked to various diseases such as neurodegenerative diseases, inflammatory disorders and cancers. Autophagy is characterized by the formation of double-membrane vesicles called autophagosomes, which engulf the cytoplasmic contents to lysosomes for destruction. It is a multistep process governed by multiple complexes. The ULK1/2 complex is one of the complexes, which is essential for autophagy initiation. It consists of the serine/threonine kinase ULK1/2, ATG13, RB1CC1/FIP200, and ATG101. Despite the extensive studies on the mechanism of autophagy, it remains ambiguous how the autophagy initiation and autophagosome formation are regulated.
An E3 ubiquitin ligase complex, linear ubiquitin chain assembly complex (LUBAC) and a de-ubiquitinating enzyme (DUB) OTULIN (OTU deubiquitinase with linear linkage specificity) are newly identified enzymes that can specific modified linear ubiquitination. Dysregulation of linear ubiquitin is linked to a number of severe auto-inflammation diseases. Liu’s group found LUBAC and OTULIN localize to the phagophore area to control autophagy initiation and maturation. LUBAC key component RNF31/HOIP translocates to the LC3 puncta area when autophagy is induced. RNF31 knockdown inhibits autophagy initiation, and cells are more sensitive to bacterial infection. OTULIN knockdown, however, promotes autophagy initiation but blocks autophagy maturation. In OTULIN knockdown cells, excessive ubiquitinated ATG13 protein was recruited to the phagophore for prolonged expansion, and therefore inhibits autophagosome maturation. Together, their study provides evidence that LUBAC and OTULIN cooperatively regulate autophagy initiation and autophagosome maturation by mediating the linear ubiquitination and the stabilization of ATG13. This work, for the first time, reports that linear ubiquitination is involved in regulating autophagy pathway, and opens a new door for understanding autophagy and inflammatory disorders.
This study was supported by the National Natural Science Foundation of China and the Start-up grant from ShanghaiTech University.
Link: https://www.tandfonline.com/doi/full/10.1080/15548627.2020.1781393
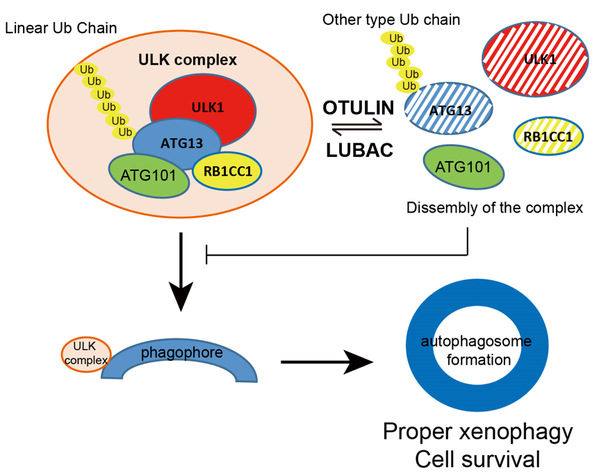
Figure: A working model of LUBAC and OTULIN mediating linear ubiquitination on ATG13 to regulate autophagy initiation.

