iHuman Institute and SLST Professor Shui Wenqing’s group published a review in Current Opinion in Biotechnology in October 2019, “Systematic mapping of protein-metabolite interactions with mass spectrometry-based techniques”. This review presents a comprehensive overview of chemical proteomics techniques developed for probing protein-metabolite interactions in the cell. Shui Wenqing’s group has been focusing on developing mass spectrometry (MS)-based techniques for identification of novel bioactive ligands towards drug targets, especially GPCRs. Earlier this year, they established an iterative affinity mass spectrometry technology which was applied to high-throughput screening of chemical modulators for GPCR targets. The study was published in Analytical Chemistry in July 2019, entitled “Accelerating the Throughput of Affinity Mass Spectrometry-Based Ligand Screening toward a G Protein-Coupled Receptor”.
Molecular recognition between proteins and small molecule metabolites plays an essential role in regulating protein functions and controlling various cellular processes. The activities of metabolic enzymes, transcription factors, trans-porters and membrane receptors can be all mediated by protein–metabolite interactions (PMIs), thus connecting cellular metabolism to genetic/epigenetic regulation, environmental sensing and signal transduction. It has been postulated that millions of functionally relevant PMIs may occur in bacterial cells, which compose equivalent complexity, if not more, compared to protein–protein inter-actions (PPIs) on the proteome scale. Up till now, the most comprehensive PMI analysis resulted in identification of 1678 interaction pairs between 20 designated metabolites and over 800 cellular proteins in Escherichia coli cells whereas a network of more than 56,000 putative PPIs was constructed in mammalian cells. Technical hurdles in large-scale mapping of intracellular PMIs mainly reside in: 1) difficulty of detecting low-affinity and transient PMIs, 2) in vitro purification not feasible for certain proteins especially full-length transmembrane proteins.
Recently, the rapid advance of mass spectrometry (MS)-based proteomics and metabolomics technology has been constantly promoting systematic profiling of PMIs in the cell. The article reviews recent progress in exploiting proteomic or metabolomic strategies for systematic PMI mapping, and the strengths and limitations of different PMI mapping approaches as well as the key elements in MS quantification and data mining is further discussed (Fig. 1). This review summarizes valuable strategies for exploring uncharacterized PMIs that may reveal new interaction-derived functionality and disease associations.
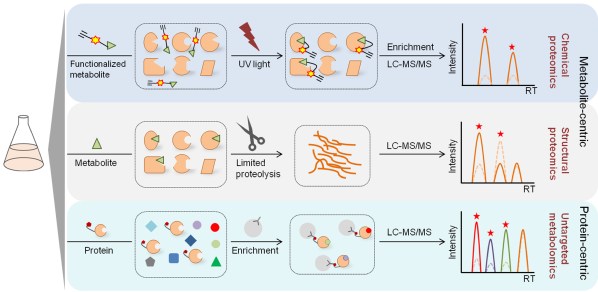
Fig. 1 Systematic mapping of PMIs with metabolite-centric or protein-centric strategies
Affinity mass spectrometry (MS) described in this review enables rapid screening of compound mixtures for ligands associated with a given protein target. However, the current screening throughput of affinity MS is limited to assaying 400-2000 compounds in one pool at a time. Furthermore, affinity MS-based screen usually requires purification of stable and active proteins, posing a great challenge for membrane receptor targets. Based on previous research published by Shui group (Chem. Sci. 2018), the researchers have developed an iterative affinity MS approach that allows screening of 20,000 compounds mixed in one pool to achieve highly efficient ligand discovery towards a GPCR target. Quantitative measurement of compound binding to the receptor enables high-affinity ligand selection using both the purified receptor and receptor-embedded cell membranes. This high-throughput, label-free and quantitative affinity MS screen resulted in discovery of three new antagonists of the A2A adenosine receptor. This work was published recently Analytical Chemistry.
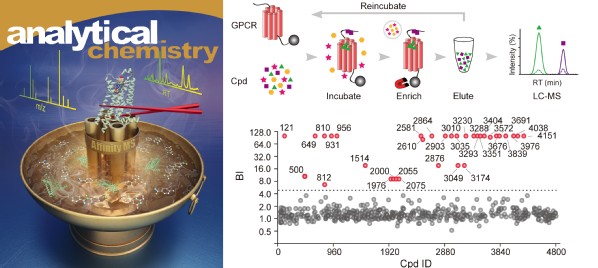
Fig. 2 Accelerating GPCR ligand screening by the newly developed iterative affinity MS approach
Li Shanshan, research assistant at iHuman Institute, is the first author of this review and Professor Shui Wenqing is the corresponding author. Graduate student Lu Yan and research assistant Qin Shanshan are co-first authors of the affinity MS research publication, Professor Shui Wenqing and Professor Wang Mingwei are corresponding authors. This work was supported by startup funding from ShanghaiTech University, National Key R&D Program of China and National Natural Science Foundation of China.

