Understanding adsorption behavior in porous crystals is critical for their rational design and performance optimization at the molecular level. A collaborative team led by ShanghaiTech University Professor Osamu Terasaki has developed “gas adsorption crystallography” for tracing adsorbates in each pore of porous crystals even with intricate pore structures.
As a collective phenomenon, adsorption isotherm provides the overall amount of gas or vapor take up by porous materials at each equilibrium pressure. However, no direct information about the location of adsorbates and their arrangements is given. Especially, for metal-organic frameworks (MOFs) having heterogeneous surface and multiple pore environment, it is even more difficult to precisely simulate their allocation considering the framework flexibility and changing of total energy minimum upon adsorption. With the help of in-situ X-ray diffraction, gas adsorption crystallography reveals responses of crystal through unit cell parameter and line width of each reflection, and provides total electron charge distribution, positions and numbers, contributed from both adsorbates and the host crystal through integrated intensity of each reflection. With the gas adsorption crystallography established, the collaborative team has been working on functional nanoporous crystals such as zeolites, mesoporous silica and MOFs for understanding their adsorption behavior at molecular level (O. M. Yaghi, O. Terasaki, et al. Nature, 2015, 527, 503).
In this study, they extend this approach to nanporous functional materials having multiple pores with different geometry. Two MOFs, PCN-224 and ZIF-412 with two and three pores with different geometry, pore opening and diameter were selected as the representative models (Fig. 1). Based on crystallography, different adsorption steps in each pore of MOFs could be separated and analysed. As a result, hidden events hardly accessible in the general analysis of the overall isotherms was clearly demonstrated by electron density maps through gas adsorption crystallography, such as the sequential pore filling in channel and intersection pores in PCN-224, which is difficult to distinguish in overall isotherm of PCN-224 showing single pore filling step (Fig. 2). This method provides quantitative and positional information of gas molecules in individual pores in MOFs at any stage throughout the entire adsorption and desorption process. The study of adsorption using different gas probes, such as Ar, N2 and CO2, makes it possible to unveil the geometrical impact of each pore type on adsorption behavior of different adsorbates. Specifically, the quantitative study about correlation between pore geometry and gas uptake in multilayer adsorption process allows scientists to identify the pore geometry best suited for gas storage, one of the major applications of MOFs (Fig. 3). The joint team believes that this study can be applicable to a large variety of MOFs as well as other porous materials with hierarchically arranged pores. Moreover, it will be helpful to scientists working on computation and modelling as well as chemists for the rational design of porous materials.
These results have been recently published in Nature Chemistry, in an article entitled “Isotherms of individual pores by gas adsorption crystallography.” This work was supported by CħEM, SPST of ShanghaiTech University and National Natural Science Foundation of China.
Read more: https://www.nature.com/articles/s41557-019-0257-X
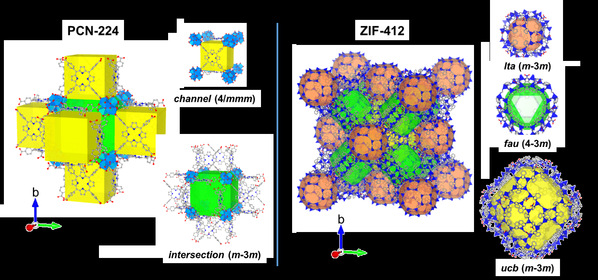
Figure 1. Structure of two MOFs, PCN-224 and ZIF-412
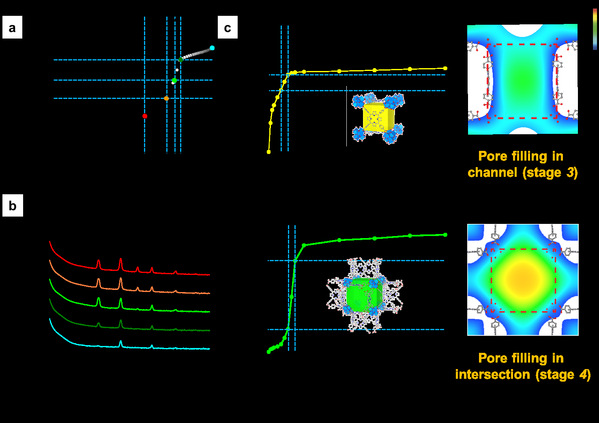
Figure 2. (a) Overall Ar adsorption isotherm of PCN-224 at 87 K (b) X-ray diffraction profiles of PCN-224 at different Ar pressures. (c) Sub-isotherms of channel and intersection of PCN-224 with electron density maps of Ar in each pore during capillary condensation.
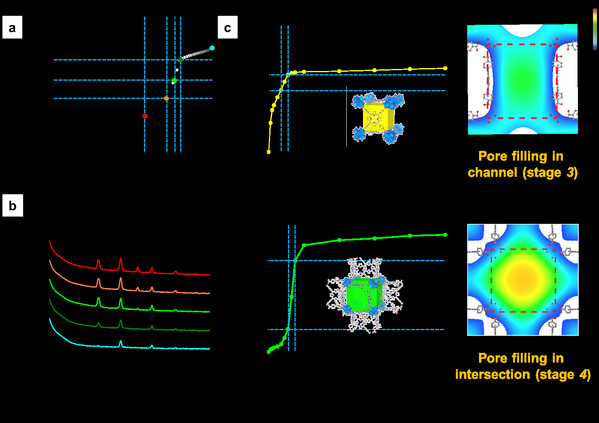
Figure 3. (a) Correlation between pore geometry (diameter and opening) of individual pores and their corresponding accumulated density of Ar. (b) Correlation between pore geometry and corresponding accumulated density of CO2. (c) Schematic illustration of the correlation between the adsorption gradients of individual pores and their contribution to the working capacity in gas storage applications. (d) Comparison of the adsorption gradients of various adsorbates in individual pores within and between MOFs.

