The Zhong group in SPST's Materials and Physical Biology Division has recently made important progress in the development of living functional materials based on engineered Bacillus subtilis biofilms. Their work, Programmable and printable Bacillus subtilis biofilms as engineered living materials, was published online in Nature Chemical Biology on Dec 3, 2018.
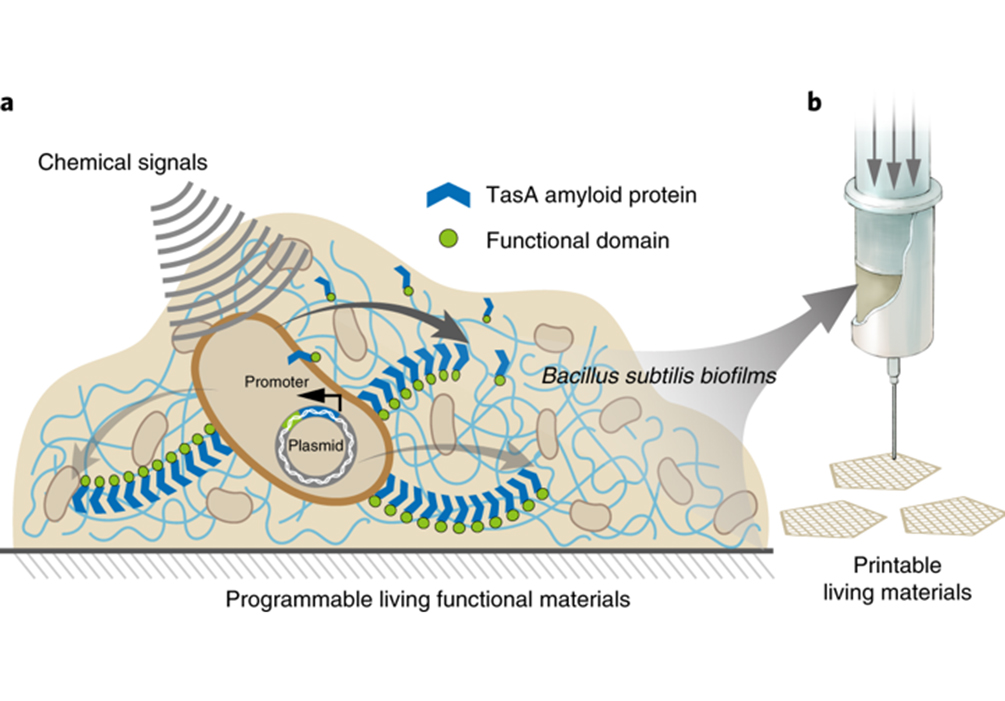
Figure 1. Design for a programmable and printable B. subtilis biofilm production platform.
Bacterial biofilms are microorganism communities encased in their secreted extracellular matrix, including proteins, exopolysaccharides, and DNAs. Previously, researchers have explored E. coli biofilms for the design of living functional materials exhibiting many attractive attributes including genetic programmability, configurable functions, and environmental responsiveness. However, E. coli export machinery’s inability to secrete large proteins significantly reduces the scope of possible material functionality. In addition, difficulties with the controlled processing of such complex materials into customizable 3D structures with well-defined designed geometries also impede their practical application. Last but not least, considering that these materials are living, and given the potential risks that live bacteria present to human beings and the environment, it is now recognized that in addition to using bacterial species that are considered safe, the careful packaging of such materials into confined environments is a particularly important and necessary design element. To address these issues, the researchers sought to utilize Bacillus subtilis, a “generally regarded as safe” (GRAS) gram-positive aerobic bacterium containing only one outer membrane, as a host to design living functional materials.
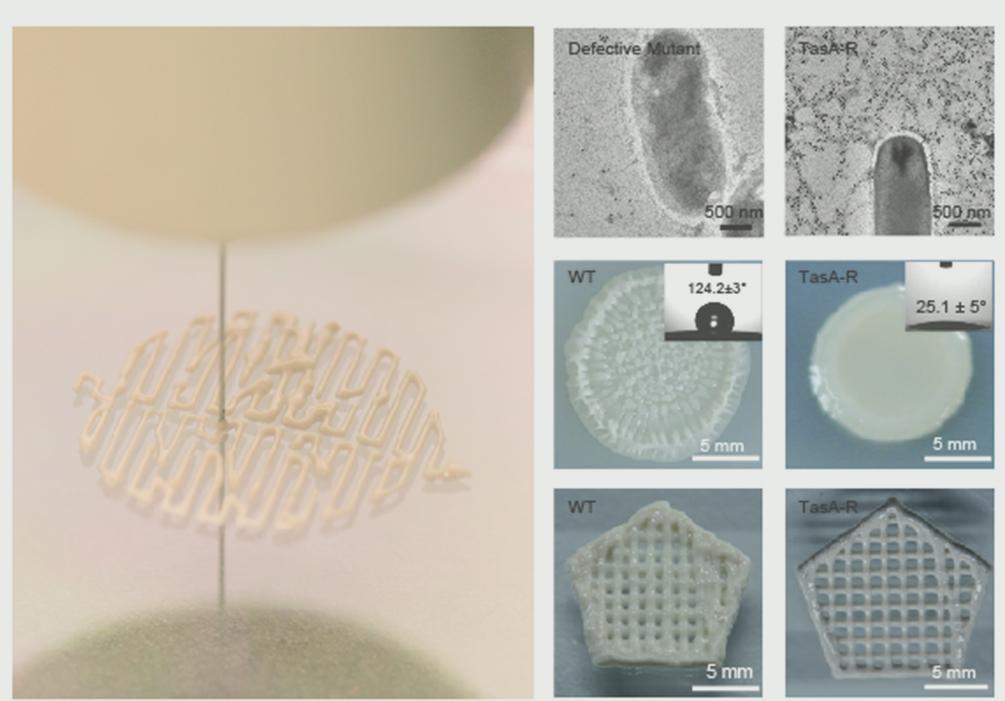
Figure 2. Printable living materials based on genetically engineered Bacillus subtilis biofilms.
The researchers first leveraged genetic engineering to develop programmable TasA fusion proteins containing a variety of functional domains or proteins that are rationally fused at the C-terminus of TasA amyloid protein (the major protein of the B. subtilis biofilm matrix). These fusion proteins could be secreted extracellularly, self-assembled into nanofibers and exhibited new functional properties as designed. As a proof-of-concept application, this programmable platform could be used for multistep reactions (i.e., biocatalytic cascades) to degrade organo-phosphate pesticides into harmless chemicals. To such ends, they chose a two-step cascade consisting of (i) the organophosphate hydrolase (OPH)-catalyzed degradation of the pesticide paraoxon (PAR) into the less harmful intermediate product paranitrophenol (PNP), and (ii) a reaction catalyzed by HisTag-immobilized gold NPs (AuNPs) in which PNP is further degraded into harmless p-aminophenol (PAP).
They further exploited the intrinsic viscoelastic properties of the engineered biofilms and fabricated well-defined ‘living shapes’ by trapping these materials into hydrogels and microgels using 3D printing and microencapsulation techniques (Fig. 4). Finally, as these bacterial biofilms are composed of living cells in well-defined geometries, they evaluated their self-regeneration capacity, storage, and long-term viability. This study demonstrates that they constructed a programmable and printable living functional material platform that may enable new applications in nanomanufacturing, biocatalysis, biomedicine, and other technical fields.
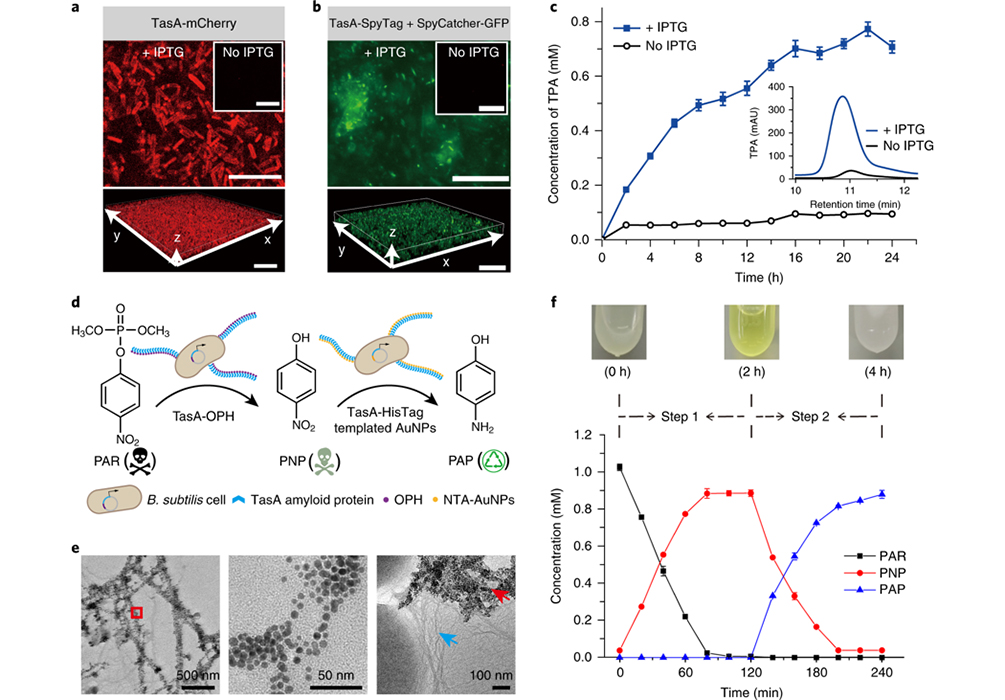
Figure 3. Functional characterization of engineered B. subtilis biofilms.

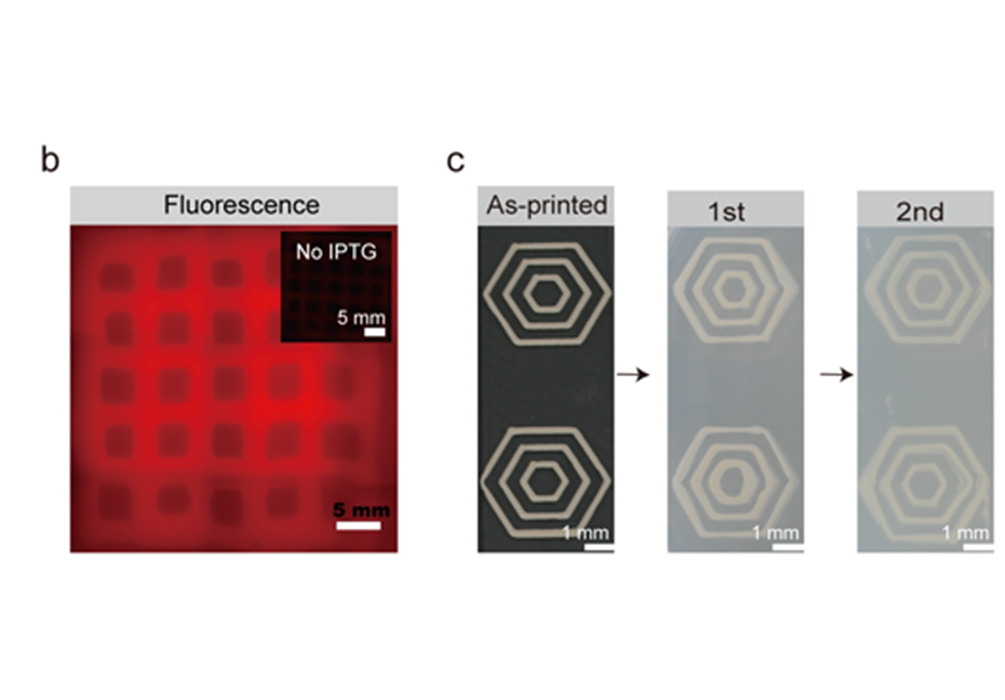
Figure 4. 3D printing, self-regeneration of engineered biofilms, and functional performance of hydrogel-trapped living B. subtilis biofilms.

Co-authors Dr. Jiaofang Huang, Suying Liu, and Chen Zhang contributed equally to this work. Professor Chao Zhong is the corresponding author. Other authors include Xinyu Wang, Jiahua Pu, Fang Ba, Shuai Xue, Prof. Haifeng Ye (East China Normal University, Shanghai, China), Tianxin Zhao, Ke Li, Yanyi Wang, Jicong Zhang, Professor Lihua Wang, Professor Chunhai Fan (Shanghai Institute of Applied Physics, CAS, and Shanghai Jiao Tong University, Shanghai, China.) and Professor Timothy K. Lu (Massachusetts Institute of Technology, Cambridge, MA, USA.). The original strain in this project was gifted from D. Kearns, Indiana University. Chinese and international patents (CN/201611156490.X and PCT/CN2018/100538) have been applied for the work related to this paper. This work was funded by the Science and Technology Commission of Shanghai Municipality (17JC1403900), National Natural Science Foundation of China (No. 31570972, No.31872728), 2016 Open Financial Fund of Qingdao National Laboratory for Marine Science and Technology (Grant No. QNLM2016ORP0403) and start-up funding support from ShanghaiTech University.
The first co-authors and corresponding author (from left to right: Miss Suying Liu, Dr. Chao Zhong, Dr. Jiaofang Huang and Mr. Chen Zhang.)
Read more at: https://www.nature.com/articles/s41589-018-0169-2

