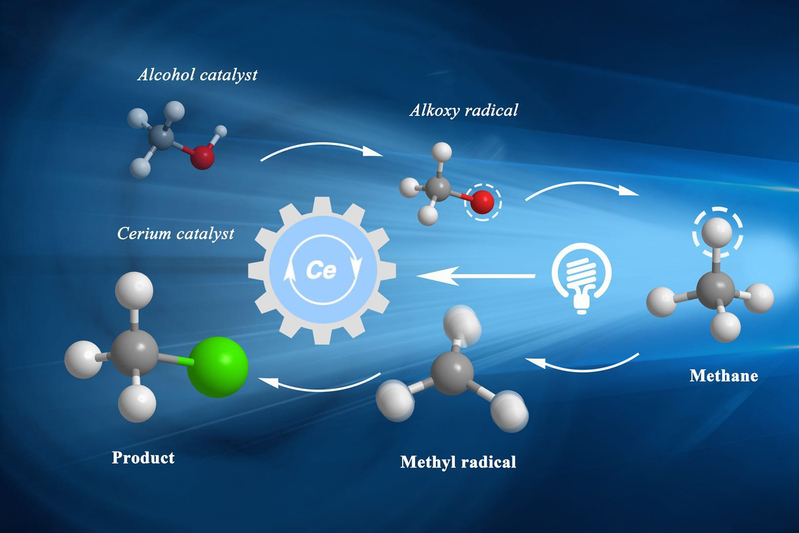
The Zuo Group at the School of Physical Science and Technology recently developed a photocatalytic methane conversion methodology which can directly transform methane, ethane and other gaseous alkanes into value-added liquid product. This breakthrough in organic chemistry provides a novel, green and mild catalytic platform for natural gas utilization, and could lead to broad applications in the energy/chemical industry. Their result was published as “Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis” in Science on July 27th. Postdoctoral researchers Anhua Hu and Jingjing Guo are co-first authors, graduate student Hui Pan is the second author, and Zuo Zhiwei is the corresponding author.
Methane and other gaseous alkanes have been traditionally viewed more as clean energy fuels than economical chemical feedstocks by the chemical community. With dwindling oil supplies and the growing importance of reducing worldwide dependence on petroleum-based chemical products, the recent discovery of huge volumes of unconventional reservoirs and soaring production of natural gas has made these gaseous hydrocarbons economically attractive and strategically important basic raw materials. The intrinsic inertness of C–H bond in methane and other gaseous alkanes has, however, brought extreme challenges for catalytic systems. These challenges are not only in the activation step, but also in controlling chemoselectivity to avoid solvent functionalization and overfunctionalization under frequently utilized harsh conditions (high temperature, superacids or strong oxidants). Moreover, the gaseous substrates’ low solubility in most solvents has raised substantial practical difficulties. Elegant catalytic systems utilizing transition metals such as Pd, Ir, Rh, Ru have been reported; however, the “grand challenge” remains the development of efficient catalytic systems with inexpensive catalysts and ambient conditions.
The Zuo group has been focused on the development of sustainable catalyst for highly efficient transformations. The unique electron structure of high valence cerium complexes, as well as their unique photophysical properties, attracted their research attention to explore valuable synthetic methodologies utilizing the ligand-to-metal charge transfer (LMCT) excitation process, a common photoexcitation manifold among coordination complexes of transition metal with an empty valence shell which has been under-investigated in synthetic organic transformations via modern photoredox catalysis. In 2016, they first found that CeCl3 could act as photocatalyst in the C-C bond cleavage and amination of cycloalkanols. Then, in 2017, they demonstrated that the LMCT process could be utilized with 1,5-HAT event for the selective distal C-H functionalization of primary alcohols. On the basis of this work, after 2202 trials and optimizations, they have developed a general and highly efficient platform for the catalytic functionalization of methane and other gaseous alkanes under LED irradiation at ambient temperature with abundant and inexpensive cerium salts as photocatalysts. Critically, the use of LMCT catalysis to generate highly reactive alkoxy radicals enables the challenging HAT event from the strong C–H bonds of the light alkanes employed. This photocatalytic platform has enabled a number of direct transformations of methane and other gaseous hydrocarbons, including amination, alkylation, and arylation, and offers intriguing opportunities for further functionalization of feedstock alkanes.
Professor Kuiling Ding, Chinese Academy of Sciences (CAS) academician and dean of Shanghai Institute of Organic Chemistry at CAS, said, “The direct functionalization of C–H bond in methane is one of the basic chemical transformations in energy and chemical processes. The high stability and low polarity of the C–H bond has brought extreme challenges for methane functionalization, therefore harsh conditions such as high temperature and high pressure are often required. The C–H functionalization of methane under mild conditions is considered a “holy grail” in the chemistry community. Through the exquisite design of the photocatalytic system, this work by the Zuo group showcases a new breakthrough in methane conversion at room temperature, and provides a new pathway for the extensive utilization of methane feedstock.”
Professor David MacMillan, one of the pioneers of modern photoredox catalysis, member of the National Academy of Sciences (USA), distinguished professor at Princeton University said, “The results of this study by the Zuo group are simply astonishing. Over the last decade, there have been many new directions arising from photoredox with significant societal impact. This study introduces a new direction (LMCT) wherein alkanes such as methane and ethane can undergo direct amination. The potential for use in sectors such as pharmaceuticals, agrochemical, and fine chemical, among others, are clearly evident. This is a remarkable paper from a young Chinese chemist that will be widely influential on a global scale. I cannot wait to see what he will do next.”
Experts from Shell, senior principal scientist Alexander van der Made and program lead methane to product Sander Van Bavel both spoke highly of the paper, “This paper on photocatalytic functionalization of alkanes showcases excellent and intriguing chemistry on the very relevant topic of alkane activation. Moreover, the paper presents a key first step towards a green route to activate alkanes under mild conditions. Ultimately, this route could lead to more extensive use of abundantly available natural gas as feedstock by chemical industry.”
“ShanghaiTech University has been striving to construct an independent and innovative academic atmosphere with full academic freedom, allowing our PIs to release their energy and creativity to the greatest extent. The breakthrough of the Zuo Group is a positive demonstration. The research group creatively used the unique rare-earth resources of China to solve the key scientific problem of methane activation, which has great importance for China and the world, in a very short period of time.” said Peidong Yang, Founding Dean of School of Physical Science and Technology, member of the National Academy of Sciences (USA) and professor at University of California, Berkeley.
This work was funded by the National Natural Science Foundation of China (21772121).