A research team led by Professor Chen Gang of the School of Physical Science and Technology at ShanghaiTech University has solved the long-standing mystery of the origin of the attractive forces between like-charged colloidal particles. Their work was recently published in the prestigious journal Nature Communications.
Colloids adsorbed at a fluid interface are ubiquitous in nature and central to a promising route to materials synthesis that combines considerable freedom of material choices with the opportunity to create highly ordered structures on the length scale from nano- to micrometers. If the interface area is confined, the repulsive Coulomb interactions between colloids can induce ordering and even crystallization. However, metastable crystallites and voids have also been observed without area confinement. Neither should be possible in a system with purely repulsive interactions, suggesting that like-charged particles at interfaces can also experience attractive interactions. Since the first direct microscopic observation of 2D colloidal crystals trapped at the air/water interface by Pieranski, considerable experimental and theoretical efforts have been devoted to the study of colloidal particle interaction, dynamics and assembly at liquid interfaces. Despite some new developments in experiments and the emergence of several theories in recent decades, much confusion still surrounds the interfacial crystallization process and the origin of the attractive forces between like-charged colloidal particles.
In this work, Professor Chen and his team report the in situ grazing-incidence small-angle X-ray scattering (GISAXS) study of the real-time self-assembly process of polystyrene nanospheres on the air/water interface of a Langmuir-Blodgett (LB) trough. This approach allows for simultaneous monitoring of the in-plane ordering and crystallization and the out-of-plane immersion depth (or contact angle) variation, providing a complete picture of the interfacial colloidal self-assembly process. Upon compression, multiple metastable intermediate states are observed before the stable 2D hexagonal close-packed superlattice monolayer forms under van der Waals attraction. Surprisingly, the immersion depth of the colloidal particles is found to vary with the interparticle distance. Numerical simulations demonstrate that the out-of-plane component of the electrostatic force from neighboring particles increases as the interparticle distance decreases. Such a force presses the particle into water, which deforms the interface and induces the long-ranged capillary attraction. This new finding provides an important clue to the long-standing mystery of the attractive interaction between like-charged particles.
Read more:https://www.nature.com/articles/s41467-018-03767-y
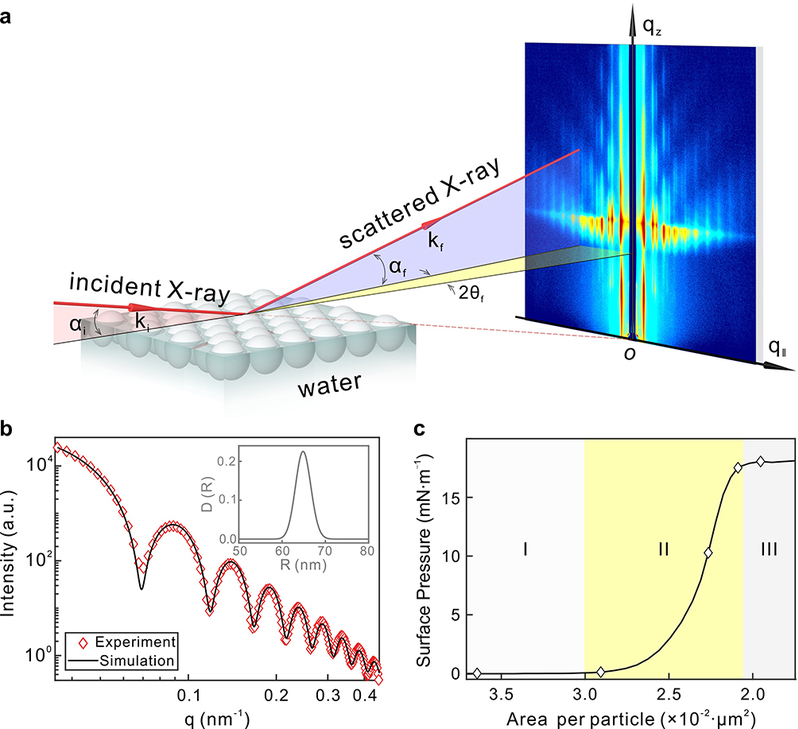
Figure 1:GISAXS experimental setup, SAXS data and surface pressure isotherm
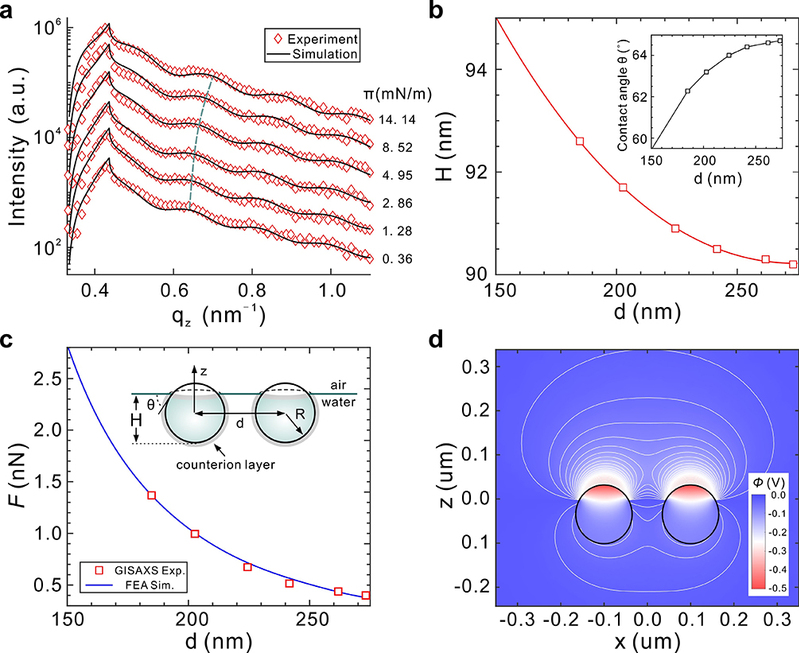
Figure 2: X-ray scattering profiles, particle positions and electrostatic interactions

