A team of scientists led by the iHuman Institute of ShanghaiTech University in collaboration with Fudan University has determined the high-resolution crystal structure of the multi-domain human smoothened receptor. The results illustrate the allosteric domain-domain interactions within the receptor, and their role in smoothened activation. These new findings are published on May 17th, 2017 in Nature Communications, titled “Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand” by Zhang X-J et al.
As a central player in the Hedgehog signaling pathway, a system that is actively involved in embryonic development and tumorigenesis, the smoothened receptor (SMO) has been long-sought after as a drug target for numerous cancers. SMO belongs to the Frizzled class of G protein-coupled receptors (GPCRs) containing a characteristic extracellular cysteine-rich domain (CRD) in addition to the canonicalseven-transmembrane helices domain (TMD). Past drug discovery efforts on SMO has yielded many effective anti-tumor agents. Yet problems with the long-term usage of these drugs have been seen as the development of drug resistance due to mutations in SMO. All these drugs bind to the orthosteric ligand binding site on the TMD. “Development of next-generation anti-SMO drugs will be facilitated by understanding of the multi-domain arrangement in the SMO structure,” said Fei Xu, Principal Investigator of iHuman Institute and Assistant Professor of School of Life Science and Technology, ShanghaiTech University, and the lead corresponding author of this paper. “This structure will allow us to identify potentially new ligand binding sites and signaling mechanisms.”
"To stabilize the multi-domain human SMO protein for crystallization, we designed a series of tool ligands," said Houchao Tao, Research Associate Professor at iHuman Institute. "TC114 is a probe that significantly stabilizes and locks the receptor into a single conformation." With further optimization of protein engineering, expression, purification, and crystallization conditions, Xianjun Zhang—a PhD student at ShanghaiTech University and the first author of this paper—finally solved the multi-domain SMO structure bound to TC114 at 2.9 Angstrom using x-ray free electron laser. “In addition to the CRD and TMD, this structure also reveals a third domain—the hinge domain (HD) that may play important modulating roles on not only connecting the CRD and TMD, but facilitating the signal transduction from the extracellular to the intracellular side on SMO as well,” said Xianjun Zhang. The role of the HD is further assessed by mutagenesis and pharmacological analysis carried out by the Tan group at Fudan University. "Identification of the HD may provide hints for the development of new modulators targeting on this region," the authors said.
"This is beautiful teamwork," said Raymond Stevens, Founding Director of iHuman Institute, ShanghaiTech University. "Chemistry and biology are bridged together in this science to understand the structure and function of this complex multi-domain receptor. The crystal structure, in turn, opens new avenues for drug discovery."
Other co-authors of this paper are Fei Zhao, Yiran Wu, Suwen Zhao, Lintao Ye, Xi Lin, Kang Ding from ShanghaiTech University, Jun Yang from Fudan University, Gye Won Han, Andrii Ishchenko and Vadim Cherezov from the University of Southern California, Venkatasubramanian Dharmarajan and Patrick R. Griffin from The Scripps Research Institute, Cornelius Gati from Medical Research Council, Garrett Nelson from Arizona University, Mark S. Hunter from Linac Coherent Light Source, and Michael A. Hanson from GPCR Consortium.Financial support for this work comes from, in part,Shanghai Municipal Government, ShanghaiTech University, and Shanghai "Pujiang Talents" grant.
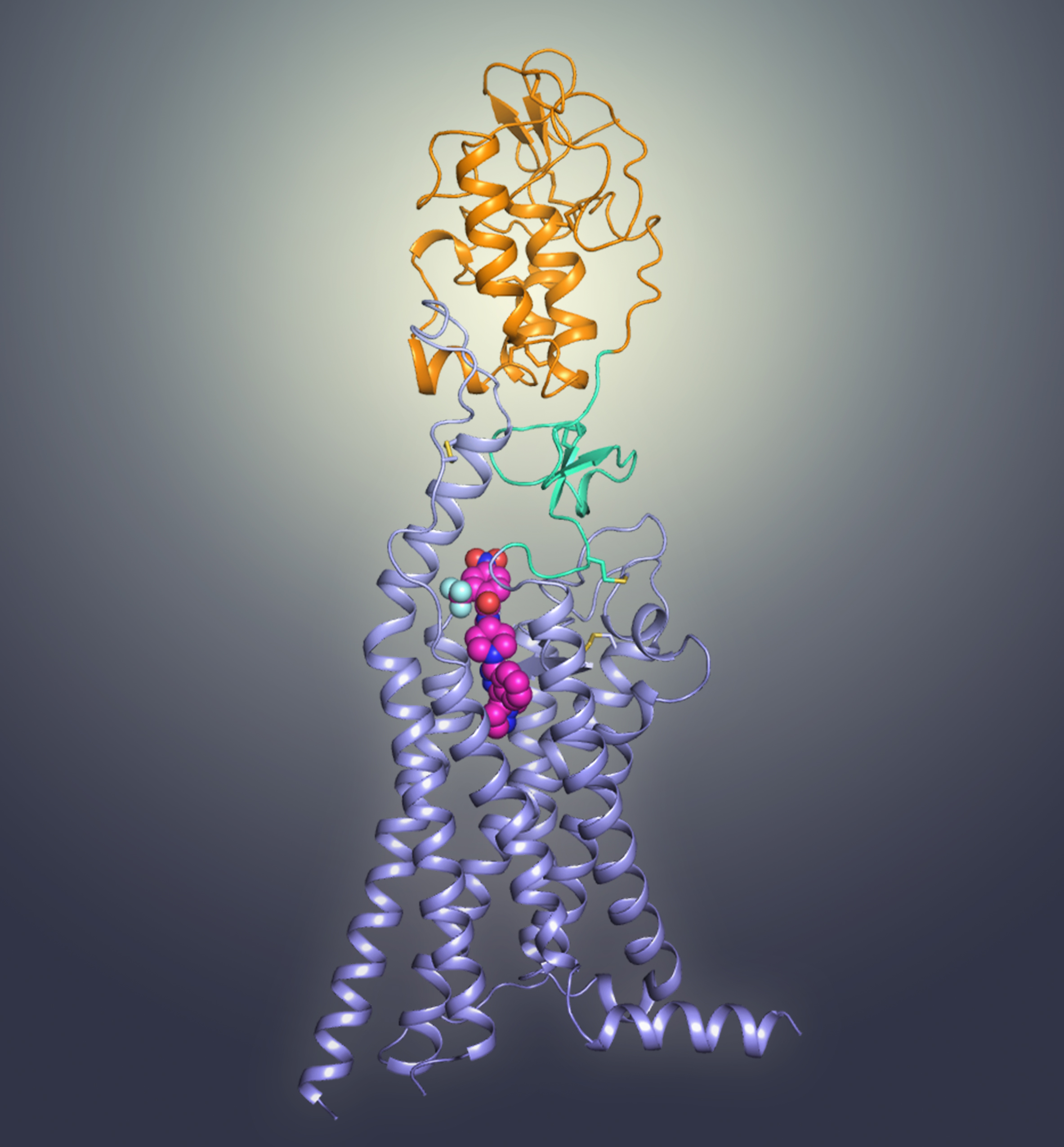
The crystal structure of multi-domain human Smoothened receptor (PDB ID: 5V56). The cysteine-rich domain (CRD), transmembrane domain (TMD) and hinge domain (HD) are shown in orange, light blue, and green, respectively. The ligand TC114 is shown as spheres in magenta.

The iHuman team from left to right: Fei Zhao, Lintao Ye, Suwen Zhao, Houchao Tao, Xianjun Zhang, Fei Xu, Yiran Wu, Kang Ding, Xi Lin.
- About
- News
- Faculty
- Research
-
Academics
- School of Physical Science and Technology (SPST)
- School of Life Science and Technology (SLST)
- School of Information Science and Technology (SIST)
- School of Entrepreneurship and Management (SEM)
- School of Creativity and Art (SCA)
- Institute of Humanities (IH)
- School of Biomedical Engineering (BME)
- Shanghai Institute for Advanced Immunochemical Studies (SIAIS)
- iHuman Institute
- Institute of Mathematical Sciences (IMS)
- Center for Transformative Science (CTS)
- Institute of Carbon Neutrality (ICN)
- Shanghai Clinical Research and Trial Center
- Tech Transfer
- Global
- Campus Life

